Endocytosis of hepatitis C virus non-enveloped capsid-like particles induces MAPK-ERK1/2 signaling events
- PMID: 20358251
- PMCID: PMC11115770
- DOI: 10.1007/s00018-010-0351-5
Endocytosis of hepatitis C virus non-enveloped capsid-like particles induces MAPK-ERK1/2 signaling events
Abstract
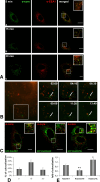
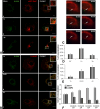
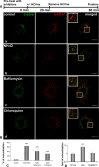
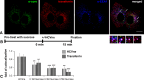
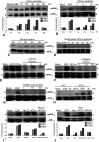
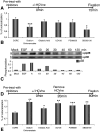
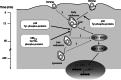
Although HCV is an enveloped virus, naked nucleocapsids have been reported in the serum of infected patients. The HCV core particle serves as a protective capsid shell for the viral genome and recombinant in vitro assembled HCV core particles induce strong specific immunity. We investigated the post-binding mechanism of recombinant core particle uptake and its intracellular fate. In hepatic cells, these particles are internalized, most likely in a clathrin-dependent pathway, reaching early to late endosomes and finally lysosomes. The endocytic acidic milieu is implicated in trafficking process. Using specific phosphoantibodies, signaling pathway inhibitors and chemical agents, ERK(1/2) was found to be activated in a sustained way after endocytosis, followed by downstream immediate early genes (c-fos and egr-1) modulation. We propose that the intriguing properties of cellular internalization of HCV non-enveloped particles can induce specific ERK(1/2)-MAPKs events that could be important in HCV life cycle and pathogenesis of HCV infection.
Figures

Similar articles
-
Modulation of IL-2 expression after uptake of hepatitis C virus non-enveloped capsid-like particles: the role of p38 kinase.Cell Mol Life Sci. 2011 Feb;68(3):505-22. doi: 10.1007/s00018-010-0466-8. Epub 2010 Jul 31. Cell Mol Life Sci. 2011. PMID: 20680391 Free PMC article.
-
Role of p38 MAPK and RNA-dependent protein kinase (PKR) in hepatitis C virus core-dependent nuclear delocalization of cyclin B1.J Biol Chem. 2006 Apr 21;281(16):10983-9. doi: 10.1074/jbc.M512536200. Epub 2006 Jan 30. J Biol Chem. 2006. PMID: 16446363
-
Clathrin-mediated endocytosis and lysosomal cleavage of hepatitis B virus capsid-like core particles.J Biol Chem. 2006 Jun 16;281(24):16563-9. doi: 10.1074/jbc.M601418200. Epub 2006 Apr 17. J Biol Chem. 2006. PMID: 16618702
-
MEK5/ERK5/mef2: a novel signaling pathway affected by hepatitis C virus non-enveloped capsid-like particles.Biochim Biophys Acta. 2011 Oct;1813(10):1854-62. doi: 10.1016/j.bbamcr.2011.06.015. Epub 2011 Jul 8. Biochim Biophys Acta. 2011. PMID: 21767578
-
Intracellular Trafficking of HBV Particles.Cells. 2020 Sep 2;9(9):2023. doi: 10.3390/cells9092023. Cells. 2020. PMID: 32887393 Free PMC article. Review.
Cited by
-
Modulation of IL-2 expression after uptake of hepatitis C virus non-enveloped capsid-like particles: the role of p38 kinase.Cell Mol Life Sci. 2011 Feb;68(3):505-22. doi: 10.1007/s00018-010-0466-8. Epub 2010 Jul 31. Cell Mol Life Sci. 2011. PMID: 20680391 Free PMC article.
-
Hepcidin and the iron enigma in HCV infection.Virulence. 2014 May 15;5(4):465-76. doi: 10.4161/viru.28508. Epub 2014 Mar 13. Virulence. 2014. PMID: 24626108 Free PMC article. Review.
-
The Epigenetic Controller Lysine-Specific Demethylase 1 (LSD1) Regulates the Outcome of Hepatitis C Viral Infection.Cells. 2023 Nov 3;12(21):2568. doi: 10.3390/cells12212568. Cells. 2023. PMID: 37947646 Free PMC article.
-
Persistent expression of hepatitis C virus non-structural proteins leads to increased autophagy and mitochondrial injury in human hepatoma cells.PLoS One. 2011;6(12):e28551. doi: 10.1371/journal.pone.0028551. Epub 2011 Dec 2. PLoS One. 2011. PMID: 22164304 Free PMC article.
-
A complex signaling network involving protein kinase CK2 is required for hepatitis C virus core protein-mediated modulation of the iron-regulatory hepcidin gene expression.Cell Mol Life Sci. 2014 Nov;71(21):4243-58. doi: 10.1007/s00018-014-1621-4. Epub 2014 Apr 10. Cell Mol Life Sci. 2014. PMID: 24718935 Free PMC article.
References
-
- Bouvier-Alias M, Patel K, Dahari H, Beaucourt S, Larderie P, Blatt L, Hezode C, Picchio G, Dhumeaux D, Neumann AU, McHutchison JG, Pawlotsky JM. Clinical utility of total HCV core antigen quantification: a new indirect marker of HCV replication. Hepatology. 2002;36:211–218. doi: 10.1053/jhep.2002.34130. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

