Toxins from bacteria
- PMID: 20358680
- PMCID: PMC3564551
- DOI: 10.1007/978-3-7643-8338-1_1
Toxins from bacteria
Abstract
Bacterial toxins damage the host at the site of bacterial infection or distant from the site. Bacterial toxins can be single proteins or oligomeric protein complexes that are organized with distinct AB structure-function properties. The A domain encodes a catalytic activity. ADP ribosylation of host proteins is the earliest post-translational modification determined to be performed by bacterial toxins; other modifications include glucosylation and proteolysis. Bacterial toxins also catalyze the non-covalent modification of host protein function or can modify host cell properties through direct protein-protein interactions. The B domain includes two functional domains: a receptor-binding domain, which defines the tropism of a toxin for a cell and a translocation domain that delivers the A domain across a lipid bilayer, either on the plasma membrane or the endosome. Bacterial toxins are often characterized based upon the secretion mechanism that delivers the toxin out of the bacterium, termed types I-VII. This review summarizes the major families of bacterial toxins and also describes the specific structure-function properties of the botulinum neurotoxins.
Figures

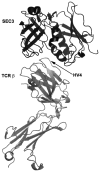
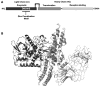

References
-
- Giannella RA. Pathogenesis of acute bacterial diarrheal disorders. Ann Rev Med. 1981;32:341–357. - PubMed
-
- Takao T, HT, Aimoto S, Shimonishi Y, Hara S, Takeda T, Takeda Y, Miwatani T. Amino acid sequence of a heat-stable enterotoxin isolated from enterotoxigentic Escherichia coli strain 18D. FEBS Lett. 1983;152:1–5. - PubMed
-
- Ozaki H, ST, Kubota H, Hata Y, Katsube Y, Shimonishi Y. Molecular structure of the toxic domain of heat-stable enterotoxin produced by enterotoxigenic Escherichia coli. J Biol Chem. 1991;266:5934–5941. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
