Is vanadate reduced by thiols under biological conditions? Changing the redox potential of V(V)/V(IV) by complexation in aqueous solution
- PMID: 20359175
- PMCID: PMC2884226
- DOI: 10.1021/ic100080k
Is vanadate reduced by thiols under biological conditions? Changing the redox potential of V(V)/V(IV) by complexation in aqueous solution
Abstract
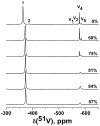
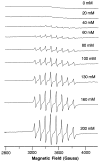
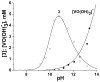
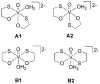
Although dogma states that vanadate is readily reduced by glutathione, cysteine, and other thiols, there are several examples documenting that vanadium(V)-sulfur complexes can form and be observed. This conundrum has impacted life scientists for more than two decades. Investigation of this problem requires an understanding of both the complexes that form from vanadium(IV) and (V) and a representative thiol in aqueous solution. The reactions of vanadate and hydrated vanadyl cation with 2-mercaptoethanol have been investigated using multinuclear NMR, electron paramagnetic resonance (EPR), and UV-vis spectroscopy. Vanadate forms a stable complex of 2:2 stoichiometry with 2-mercaptoethanol at neutral and alkaline pH. In contrast, vanadate can oxidize 2-mercaptoethanol; this process is favored at low pH and high solute concentrations. The complex that forms between aqueous vanadium(IV) and 2-mercaptoethanol has a 1:2 stoichiometry and can be observed at high pH and high 2-mercaptoethanol concentration. The solution structures have been deduced based on coordination induced chemical shifts and speciation diagrams prepared. This work demonstrates that both vanadium(IV) and (V)-thiol complexes form and that redox chemistry also takes place. Whether reduction of vanadate takes place is governed by a combination of parameters: pH, solute- and vanadate-concentrations and the presence of other complexing ligands. On the basis of these results it is now possible to understand the distribution of vanadium in oxidation states (IV) and (V) in the presence of glutathione, cysteine, and other thiols and begin to evaluate the forms of the vanadium compounds that exert a particular biological effect including the insulin-enhancing agents, antiamoebic agents, and interactions with vanadium binding proteins.
Figures
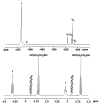

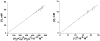

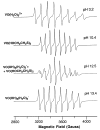
 [VO(OCH2CH2S)2]2− + 2 H+.
[VO(OCH2CH2S)2]2− + 2 H+.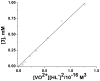

Similar articles
-
The mode of reduction of vanadate(+V) to oxovanadium(+IV) by glutathione and cysteine.Toxicology. 1986 Dec 15;42(2-3):281-9. doi: 10.1016/0300-483x(86)90016-8. Toxicology. 1986. PMID: 3026064
-
Aqueous chemistry of the vanadium(III) (V(III)) and the V(III)-dipicolinate systems and a comparison of the effect of three oxidation states of vanadium compounds on diabetic hyperglycemia in rats.Inorg Chem. 2005 Jul 25;44(15):5416-27. doi: 10.1021/ic048331q. Inorg Chem. 2005. PMID: 16022540
-
Chromatographic and spectrophometric studies of vanadate (+V) reduction by 3-mercaptopropionic acid.J Inorg Biochem. 2022 May;230:111747. doi: 10.1016/j.jinorgbio.2022.111747. Epub 2022 Feb 3. J Inorg Biochem. 2022. PMID: 35151102
-
The (biological) speciation of vanadate(V) as revealed by (51)V NMR: A tribute on Lage Pettersson and his work.J Inorg Biochem. 2015 Jun;147:25-31. doi: 10.1016/j.jinorgbio.2014.12.014. Epub 2014 Dec 19. J Inorg Biochem. 2015. PMID: 25592749 Review.
-
Vanadium. Its role for humans.Met Ions Life Sci. 2013;13:139-69. doi: 10.1007/978-94-007-7500-8_5. Met Ions Life Sci. 2013. PMID: 24470091 Free PMC article. Review.
Cited by
-
Anti-diabetic effects of a series of vanadium dipicolinate complexes in rats with streptozotocin-induced diabetes.Coord Chem Rev. 2011 Oct;255(19-20):2258-2269. doi: 10.1016/j.ccr.2011.06.015. Coord Chem Rev. 2011. PMID: 23049138 Free PMC article.
-
Vanadyl bisacetylacetonate protects β cells from palmitate-induced cell death through the unfolded protein response pathway.J Biol Inorg Chem. 2011 Jun;16(5):789-98. doi: 10.1007/s00775-011-0780-0. Epub 2011 Apr 22. J Biol Inorg Chem. 2011. PMID: 21512771
-
Impact of Environmental Risk Factors on Mitochondrial Dysfunction, Neuroinflammation, Protein Misfolding, and Oxidative Stress in the Etiopathogenesis of Parkinson's Disease.Int J Mol Sci. 2022 Sep 16;23(18):10808. doi: 10.3390/ijms231810808. Int J Mol Sci. 2022. PMID: 36142718 Free PMC article. Review.
-
On the Capability of Oxidovanadium(IV) Derivatives to Act as All-Around Catalytic Promoters Since the Prebiotic World.Molecules. 2020 Jul 6;25(13):3073. doi: 10.3390/molecules25133073. Molecules. 2020. PMID: 32640541 Free PMC article. Review.
-
In Vitro, Oral Acute, and Repeated 28-Day Oral Dose Toxicity of a Mixed-Valence Polyoxovanadate Cluster.Pharmaceuticals (Basel). 2023 Aug 30;16(9):1232. doi: 10.3390/ph16091232. Pharmaceuticals (Basel). 2023. PMID: 37765040 Free PMC article.
References
-
- Crans DC, Zhang BY, Gaidamauskas E, Keramidas A, Roberts C. Abstracts of Papers. 223rd ACS National Meeting; Orlando, FL, United States. April 7–11, 2002; 2002. p. INOR-242.
-
- Hsu HF, Su CL, Gopal NO, Wu CC, Chu WC, Tsai YF, Chang YH, Liu YH, Kuo TS, Ke SC. Eur J Inorg Chem. 2006:1161–1167.
-
- Kawakami N, Ueki T, Amata Y, Kanamori K, Matsuo K, Gekko K, Michibata H. Biochim Biophys Acta -Prot Proteom. 2009;1794:674–679. - PubMed
-
- Ueki T, Shintaku K, Yonekawa Y, Takatsu N, Yamada H, Hamada T, Hirota H, Michibata H. Biochim Biophys Acta-Gen Subj. 2007;1770:951–957. - PubMed
-
- Singh S, Bharti N, Mohapatra PP. Chem Rev. 2009;109:1900–1947. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

