Undifferentiated primate spermatogonia and their endocrine control
- PMID: 20359909
- PMCID: PMC2896565
- DOI: 10.1016/j.tem.2010.03.001
Undifferentiated primate spermatogonia and their endocrine control
Abstract
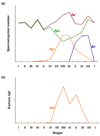
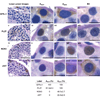
The biology of spermatogonial stem cells is currently an area of intensive research and contemporary studies in primates are emerging. Quantitative regulation of sperm output by the primate testis seems to be exerted primarily on the transition from undifferentiated to differentiating spermatogonia. This review examines recent advances in our understanding of the mechanisms governing spermatogonial renewal and early differentiation in male primates, with a focus on the monkey. Emerging revisions to the classic view of dark and pale type A spermatogonia as reserve and renewing spermatogonial stem cells, respectively, are critically evaluated and essential features of endocrine control of undifferentiated spermatogonia throughout postnatal primate development are discussed. Obstacles in gaining a more complete understanding of primate spermatogonia are also identified.
Copyright (c) 2010 Elsevier Ltd. All rights reserved.
Figures
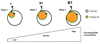
Similar articles
-
Spermatogonial stem cells in higher primates: are there differences from those in rodents?Reproduction. 2010 Mar;139(3):479-93. doi: 10.1530/REP-09-0255. Epub 2009 Oct 30. Reproduction. 2010. PMID: 19880674 Free PMC article. Review.
-
The testicular transcriptome associated with spermatogonia differentiation initiated by gonadotrophin stimulation in the juvenile rhesus monkey (Macaca mulatta).Hum Reprod. 2017 Oct 1;32(10):2088-2100. doi: 10.1093/humrep/dex270. Hum Reprod. 2017. PMID: 28938749 Free PMC article.
-
Regulation of spermatogonial proliferation.Ann N Y Acad Sci. 1989;564:140-53. doi: 10.1111/j.1749-6632.1989.tb25894.x. Ann N Y Acad Sci. 1989. PMID: 2672954 Review.
-
A-single spermatogonia heterogeneity and cell cycles synchronize with rat seminiferous epithelium stages VIII-IX.Biol Reprod. 2014 Feb 13;90(2):32. doi: 10.1095/biolreprod.113.113555. Print 2014 Feb. Biol Reprod. 2014. PMID: 24389876 Free PMC article.
-
Spermatogonial behavior in marmoset: a new generation, their kinetics and niche.Mol Hum Reprod. 2018 Jun 1;24(6):299-309. doi: 10.1093/molehr/gay017. Mol Hum Reprod. 2018. PMID: 29660000
Cited by
-
Very small embryonic-like stem cells: implications in reproductive biology.Biomed Res Int. 2013;2013:682326. doi: 10.1155/2013/682326. Epub 2013 Feb 13. Biomed Res Int. 2013. PMID: 23509758 Free PMC article. Review.
-
Single-cell transcriptomics reveals the cellular dynamics of hexafluoropropylene oxide dimer acid in exerting mouse male reproductive toxicity.J Anim Sci Biotechnol. 2025 Mar 11;16(1):42. doi: 10.1186/s40104-025-01177-x. J Anim Sci Biotechnol. 2025. PMID: 40069855 Free PMC article.
-
Reproductive Longevity Predicts Mutation Rates in Primates.Curr Biol. 2018 Oct 8;28(19):3193-3197.e5. doi: 10.1016/j.cub.2018.08.050. Epub 2018 Sep 27. Curr Biol. 2018. PMID: 30270182 Free PMC article.
-
Follicle-stimulating hormone signaling in Sertoli cells: a licence to the early stages of spermatogenesis.Reprod Biol Endocrinol. 2022 Jul 2;20(1):97. doi: 10.1186/s12958-022-00971-w. Reprod Biol Endocrinol. 2022. PMID: 35780146 Free PMC article. Review.
-
A single-cell transcriptomic landscape of mouse testicular aging.J Adv Res. 2023 Nov;53:219-234. doi: 10.1016/j.jare.2022.12.007. Epub 2022 Dec 14. J Adv Res. 2023. PMID: 36528294 Free PMC article.
References
-
- Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endo. Rev. 2001;22:764–786. - PubMed
-
- Sharpe RM. Regulation of Spermatogenesis. In: Knobil E, Neill JD, editors. The Physiology of Reproduction, Second Edition. Raven Press, Ltd.; 1994. pp. 1363–1434.
-
- de Rooij D. The spermatogonial stem cell niche. Microscopy Res. Tech. 2009;72:580–585. - PubMed
-
- Clermont Y. Kinetics of spermatogenesis in mammals: Seminiferous epithelium cycle and spermatogonial renewal. Physiological Reviews. 1972;52:198–236. - PubMed
-
- de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J. Androl. 2000;21:776–798. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

