Phospho-regulated ACAP4-Ezrin interaction is essential for histamine-stimulated parietal cell secretion
- PMID: 20360010
- PMCID: PMC2881800
- DOI: 10.1074/jbc.M110.129007
Phospho-regulated ACAP4-Ezrin interaction is essential for histamine-stimulated parietal cell secretion
Abstract
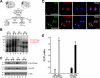
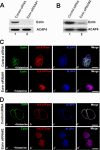
The ezrin-radixin-moesin proteins provide a regulated linkage between membrane proteins and the cortical cytoskeleton and also participate in signal transduction pathways. Ezrin is localized to the apical membrane of parietal cells and couples the protein kinase A activation cascade to the regulated HCl secretion. Our recent proteomic study revealed a protein complex of ezrin-ACAP4-ARF6 essential for volatile membrane remodeling (Fang, Z., Miao, Y., Ding, X., Deng, H., Liu, S., Wang, F., Zhou, R., Watson, C., Fu, C., Hu, Q., Lillard, J. W., Jr., Powell, M., Chen, Y., Forte, J. G., and Yao, X. (2006) Mol. Cell Proteomics 5, 1437-1449). However, knowledge of whether ACAP4 physically interacts with ezrin and how their interaction is integrated into membrane-cytoskeletal remodeling has remained elusive. Here we provide the first evidence that ezrin interacts with ACAP4 in a protein kinase A-mediated phosphorylation-dependent manner through the N-terminal 400 amino acids of ACAP4. ACAP4 locates in the cytoplasmic membrane in resting parietal cells but translocates to the apical plasma membrane upon histamine stimulation. ACAP4 was precipitated with ezrin from secreting but not resting parietal cell lysates, suggesting a phospho-regulated interaction. Indeed, this interaction is abolished by phosphatase treatment and validated by an in vitro reconstitution assay using phospho-mimicking ezrin(S66D). Importantly, ezrin specifies the apical distribution of ACAP4 in secreting parietal cells because either suppression of ezrin or overexpression of non-phosphorylatable ezrin prevents the apical localization of ACAP4. In addition, overexpressing GTPase-activating protein-deficient ACAP4 results in an inhibition of apical membrane-cytoskeletal remodeling and gastric acid secretion. Taken together, these results define a novel molecular mechanism linking ACAP4-ezrin interaction to polarized epithelial secretion.
Figures
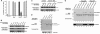


Similar articles
-
MST4 kinase phosphorylates ACAP4 protein to orchestrate apical membrane remodeling during gastric acid secretion.J Biol Chem. 2017 Sep 29;292(39):16174-16187. doi: 10.1074/jbc.M117.808212. Epub 2017 Aug 14. J Biol Chem. 2017. PMID: 28808054 Free PMC article.
-
Cell Polarity Kinase MST4 Cooperates with cAMP-dependent Kinase to Orchestrate Histamine-stimulated Acid Secretion in Gastric Parietal Cells.J Biol Chem. 2015 Nov 20;290(47):28272-28285. doi: 10.1074/jbc.M115.668855. Epub 2015 Sep 24. J Biol Chem. 2015. PMID: 26405038 Free PMC article.
-
PALS1 specifies the localization of ezrin to the apical membrane of gastric parietal cells.J Biol Chem. 2005 Apr 8;280(14):13584-92. doi: 10.1074/jbc.M411941200. Epub 2005 Jan 27. J Biol Chem. 2005. PMID: 15677456
-
[Signal transduction and intracellular recruitment of gastric proton pump in the parietal cell].Nihon Yakurigaku Zasshi. 1997 Dec;110(6):303-13. doi: 10.1254/fpj.110.303. Nihon Yakurigaku Zasshi. 1997. PMID: 9503388 Review. Japanese.
-
Membrane and protein recycling associated with gastric HCl secretion.J Intern Med Suppl. 1990;732:17-26. doi: 10.1111/j.1365-2796.1990.tb01467.x. J Intern Med Suppl. 1990. PMID: 2166524 Review.
Cited by
-
Celastrol inhibits ezrin-mediated migration of hepatocellular carcinoma cells.Sci Rep. 2020 Jul 9;10(1):11273. doi: 10.1038/s41598-020-68238-1. Sci Rep. 2020. PMID: 32647287 Free PMC article.
-
PLK1 phosphorylation of ZW10 guides accurate chromosome segregation in mitosis.J Mol Cell Biol. 2024 Jul 29;16(2):mjae008. doi: 10.1093/jmcb/mjae008. J Mol Cell Biol. 2024. PMID: 38402459 Free PMC article.
-
MST4 kinase phosphorylates ACAP4 protein to orchestrate apical membrane remodeling during gastric acid secretion.J Biol Chem. 2017 Sep 29;292(39):16174-16187. doi: 10.1074/jbc.M117.808212. Epub 2017 Aug 14. J Biol Chem. 2017. PMID: 28808054 Free PMC article.
-
Regulation of Transporters and Channels by Membrane-Trafficking Complexes in Epithelial Cells.Cold Spring Harb Perspect Biol. 2017 Nov 1;9(11):a027839. doi: 10.1101/cshperspect.a027839. Cold Spring Harb Perspect Biol. 2017. PMID: 28246186 Free PMC article. Review.
-
Phosphorylation of the Bin, Amphiphysin, and RSV161/167 (BAR) domain of ACAP4 regulates membrane tubulation.Proc Natl Acad Sci U S A. 2013 Jul 2;110(27):11023-8. doi: 10.1073/pnas.1217727110. Epub 2013 Jun 17. Proc Natl Acad Sci U S A. 2013. PMID: 23776207 Free PMC article.
References
-
- Yeaman C., Grindstaff K. K., Hansen M. D., Nelson W. J. (1999) Curr. Biol. 9, R515–R517 - PubMed
-
- Bretscher A., Edwards K., Fehon R. G. (2002) Nat. Rev. Mol. Cell Biol. 3, 586–599 - PubMed
-
- Urushidani T., Hanzel D. K., Forte J. G. (1989) Am. J. Physiol. 256, G1070–G1081 - PubMed
-
- Yao X., Forte J. G. (2003) Annu. Rev. Physiol. 65, 103–131 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

