Beneficial effect of human anti-amyloid-beta active immunization on neurite morphology and tau pathology
- PMID: 20360050
- PMCID: PMC2859150
- DOI: 10.1093/brain/awq056
Beneficial effect of human anti-amyloid-beta active immunization on neurite morphology and tau pathology
Abstract
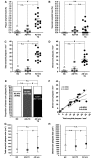
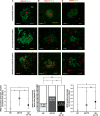
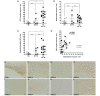
Anti-amyloid-beta immunization leads to amyloid clearance in patients with Alzheimer's disease, but the effect of vaccination on amyloid-beta-induced neuronal pathology has not been quantitatively examined. The objectives of this study were to address the effects of anti-amyloid-beta active immunization on neurite trajectories and the pathological hallmarks of Alzheimer's disease in the human hippocampus. Hippocampal sections from five patients with Alzheimer's disease enrolled in the AN1792 Phase 2a trial were compared with those from 13 non-immunized Braak-stage and age-matched patients with Alzheimer's disease, and eight age-matched non-demented controls. Analyses included neurite curvature ratio as a quantitative measure of neuritic abnormalities, amyloid and tau loads, and a quantitative characterization of plaque-associated neuritic dystrophy and astrocytosis. Amyloid load and density of dense-core plaques were decreased in the immunized group compared to non-immunized patients (P < 0.01 and P < 0.001, respectively). The curvature ratio in non-immunized patients with Alzheimer's disease was elevated compared to non-demented controls (P < 0.0001). In immunized patients, however, the curvature ratio was normalized when compared to non-immunized patients (P < 0.0001), and not different from non-demented controls. In the non-immunized patients, neurites close to dense-core plaques (within 50 microm) were more abnormal than those far from plaques (i.e. beyond 50 microm) (P < 0.0001). By contrast, in the immunized group neurites close to and far from the remaining dense-core plaques did not differ, and both were straighter compared to the non-immunized patients (P < 0.0001). Compared to non-immunized patients, dense-core plaques remaining after immunization had similar degree of astrocytosis (P = 0.6060), more embedded dystrophic neurites (P < 0.0001) and were more likely to have mitochondrial accumulation (P < 0.001). In addition, there was a significant decrease in the density of paired helical filament-1-positive neurons in the immunized group as compared to the non-immunized (P < 0.05), but not in the density of Alz50 or thioflavin-S positive tangles, suggesting a modest effect of anti-amyloid-beta immunization on tangle pathology. Clearance of amyloid plaques upon immunization with AN1792 effectively improves a morphological measure of neurite abnormality in the hippocampus. This improvement is not just attributable to the decrease in plaque load, but also occurs within the halo of the remaining dense-core plaques. However, these remaining plaques still retain some of their toxic potential. Anti-amyloid-beta immunization might also ameliorate the hippocampal tau pathology through a decrease in tau phosphorylation. These data agree with preclinical animal studies and further demonstrate that human anti-amyloid-beta immunization does not merely clear amyloid from the Alzheimer's disease brain, but reduces some of the neuronal alterations that characterize Alzheimer's disease.
Figures

Comment in
-
Are we getting to grips with Alzheimer's disease at last?Brain. 2010 May;133(Pt 5):1297-9. doi: 10.1093/brain/awq099. Brain. 2010. PMID: 20418529 No abstract available.
Similar articles
-
Relationship between apolipoprotein E and the amyloid deposits and dystrophic neurites of Alzheimer's disease.Neuropathol Appl Neurobiol. 1997 Dec;23(6):483-91. doi: 10.1111/j.1365-2990.1997.tb01325.x. Neuropathol Appl Neurobiol. 1997. PMID: 9460714
-
The morphological phenotype of beta-amyloid plaques and associated neuritic changes in Alzheimer's disease.Neuroscience. 2001;105(1):99-107. doi: 10.1016/s0306-4522(01)00169-5. Neuroscience. 2001. PMID: 11483304
-
Dissecting phenotypic traits linked to human resilience to Alzheimer's pathology.Brain. 2013 Aug;136(Pt 8):2510-26. doi: 10.1093/brain/awt171. Epub 2013 Jul 3. Brain. 2013. PMID: 23824488 Free PMC article.
-
Neuritic Plaques - Gateways to Understanding Alzheimer's Disease.Mol Neurobiol. 2024 May;61(5):2808-2821. doi: 10.1007/s12035-023-03736-7. Epub 2023 Nov 8. Mol Neurobiol. 2024. PMID: 37940777 Free PMC article. Review.
-
Amyloid-induced neurofibrillary tangle formation in Alzheimer's disease: insight from transgenic mouse and tissue-culture models.Int J Dev Neurosci. 2004 Nov;22(7):453-65. doi: 10.1016/j.ijdevneu.2004.07.013. Int J Dev Neurosci. 2004. PMID: 15465275 Review.
Cited by
-
Immunotherapy for the treatment of Alzheimer's disease: amyloid-β or tau, which is the right target?Immunotargets Ther. 2013 Dec 27;3:19-28. doi: 10.2147/ITT.S40131. eCollection 2014. Immunotargets Ther. 2013. PMID: 27471697 Free PMC article. Review.
-
Oral Immunization with Soybean Storage Protein Containing Amyloid-β 4-10 Prevents Spatial Learning Decline.J Alzheimers Dis. 2019;70(2):487-503. doi: 10.3233/JAD-190023. J Alzheimers Dis. 2019. PMID: 31177217 Free PMC article.
-
Combination Drug Therapy for the Management of Alzheimer's Disease.Int J Mol Sci. 2020 May 5;21(9):3272. doi: 10.3390/ijms21093272. Int J Mol Sci. 2020. PMID: 32380758 Free PMC article. Review.
-
Amyloid is linked to cognitive decline in patients with Parkinson disease without dementia.Neurology. 2013 Jan 1;80(1):85-91. doi: 10.1212/WNL.0b013e31827b1a07. Epub 2012 Dec 12. Neurology. 2013. PMID: 23243071 Free PMC article.
-
The biochemical aftermath of anti-amyloid immunotherapy.Mol Neurodegener. 2010 Oct 7;5:39. doi: 10.1186/1750-1326-5-39. Mol Neurodegener. 2010. PMID: 20929585 Free PMC article.
References
-
- Akiyama H, Tago H, Itagaki S, McGeer PL. Occurrence of diffuse amyloid deposits in the presubicular parvopyramidal layer in Alzheimer’s disease. Acta Neuropathol. 1990;79:537–44. - PubMed
-
- Augustinack JC, Schneider A, Mandelkow EM, Hyman BT. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 2002;103:26–35. - PubMed
-
- Bacskai BJ, Kajdasz ST, Christie RH, Carter C, Games D, Seubert P, et al. Imaging of amyloid-β deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nat Med. 2001;7:369–72. - PubMed
-
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

