Effects of treatment in women with gestational diabetes mellitus: systematic review and meta-analysis
- PMID: 20360215
- PMCID: PMC2848718
- DOI: 10.1136/bmj.c1395
Effects of treatment in women with gestational diabetes mellitus: systematic review and meta-analysis
Abstract
Objective: To summarise the benefits and harms of treatments for women with gestational diabetes mellitus.
Design: Systematic review and meta-analysis of randomised controlled trials.
Data sources: Embase, Medline, AMED, BIOSIS, CCMed, CDMS, CDSR, CENTRAL, CINAHL, DARE, HTA, NHS EED, Heclinet, SciSearch, several publishers' databases, and reference lists of relevant secondary literature up to October 2009. Review methods Included studies were randomised controlled trials of specific treatment for gestational diabetes compared with usual care or "intensified" compared with "less intensified" specific treatment.
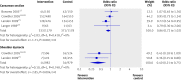
Results: Five randomised controlled trials matched the inclusion criteria for specific versus usual treatment. All studies used a two step approach with a 50 g glucose challenge test or screening for risk factors, or both, and a subsequent 75 g or 100 g oral glucose tolerance test. Meta-analyses did not show significant differences for most single end points judged to be of direct clinical importance. In women specifically treated for gestational diabetes, shoulder dystocia was significantly less common (odds ratio 0.40, 95% confidence interval 0.21 to 0.75), and one randomised controlled trial reported a significant reduction of pre-eclampsia (2.5 v 5.5%, P=0.02). For the surrogate end point of large for gestational age infants, the odds ratio was 0.48 (0.38 to 0.62). In the 13 randomised controlled trials of different intensities of specific treatments, meta-analysis showed a significant reduction of shoulder dystocia in women with more intensive treatment (0.31, 0.14 to 0.70).
Conclusions: Treatment for gestational diabetes, consisting of treatment to lower blood glucose concentration alone or with special obstetric care, seems to lower the risk for some perinatal complications. Decisions regarding treatment should take into account that the evidence of benefit is derived from trials for which women were selected with a two step strategy (glucose challenge test/screening for risk factors and oral glucose tolerance test).
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures




Comment in
-
Treatment of gestational diabetes.BMJ. 2010 Apr 1;340:c1708. doi: 10.1136/bmj.c1708. BMJ. 2010. PMID: 20360217 No abstract available.
-
ACP Journal Club: treatment for gestational diabetes reduces risk for shoulder dystocia.Ann Intern Med. 2010 Sep 21;153(6):JC3-6. doi: 10.7326/0003-4819-153-6-201009210-02006. Ann Intern Med. 2010. PMID: 20855795 No abstract available.
References
-
- Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 1998;21(suppl 2):161-7S. - PubMed
-
- Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991-2002. - PubMed
-
- Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009;373:1773-9. - PubMed
-
- Hillier TA, Vesco KK, Pedula KL, Beil TL, Whitlock EP, Pettitt DJ. Screening for gestational diabetes mellitus: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2008;148:766-75. - PubMed
-
- Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care 2007;30(suppl 2):141-6S. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
