ATF3-mediated epigenetic regulation protects against acute kidney injury
- PMID: 20360311
- PMCID: PMC2900964
- DOI: 10.1681/ASN.2009070690
ATF3-mediated epigenetic regulation protects against acute kidney injury
Abstract
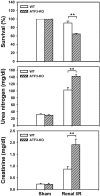
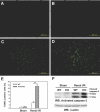
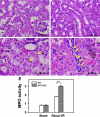
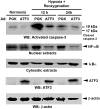
A variety of stress stimuli, including ischemia-reperfusion (I/R) injury, induce the transcriptional repressor ATF3 in the kidney. The functional consequences of this upregulation in ATF3 after renal I/R injury are not well understood. Here, we found that ATF3-deficient mice had higher renal I/R-induced mortality, kidney dysfunction, inflammation (number of infiltrating neutrophils, myeloperoxidase activity, and induction of IL-6 and P-selectin), and apoptosis compared with wild-type mice. Furthermore, gene transfer of ATF3 to the kidney rescued the renal I/R-induced injuries in the ATF3-deficient mice. Molecular and biochemical analysis revealed that ATF3 interacted directly with histone deacetylase 1 (HDAC1) and recruited HDAC1 into the ATF/NF-kappaB sites in the IL-6 and IL-12b gene promoters. The ATF3-associated HDAC1 deacetylated histones, which resulted in the condensation of chromatin structure, interference of NF-kappaB binding, and inhibition of inflammatory gene transcription after I/R injury. Taken together, these data demonstrate epigenetic regulation mediated by the stress-inducible gene ATF3 after renal I/R injury and suggest potential targeted approaches for acute kidney injury.
Figures





References
-
- Schiffl H, Lang SM, Fischer R: Daily hemodialysis and the outcome of acute renal failure. N Engl J Med 346: 305–310, 2002. - PubMed
-
- Star RA: Treatment of acute renal failure. Kidney Int 54: 1817–1831, 1998. - PubMed
-
- Daemen MA, de Vries B, Buurman WA: Apoptosis and inflammation in renal reperfusion injury. Transplantation 73: 1693–1700, 2002. - PubMed
-
- Cheung P, Allis CD, Sassone-Corsi P: Signaling to chromatin through histone modifications. Cell 103: 263–271, 2000. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

