Understanding neuronal connectivity through the post-transcriptional toolkit
- PMID: 20360381
- PMCID: PMC2849119
- DOI: 10.1101/gad.1907710
Understanding neuronal connectivity through the post-transcriptional toolkit
Abstract
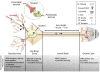
Post-transcriptional regulatory mechanisms have emerged as a critical component underlying the diversification and spatiotemporal control of the proteome during the establishment of precise neuronal connectivity. These mechanisms have been shown to be important for virtually all stages of assembling a neural network, from neurite guidance, branching, and growth to synapse morphogenesis and function. From the moment a gene is transcribed, it undergoes a series of post-transcriptional regulatory modifications in the nucleus and cytoplasm until its final deployment as a functional protein. Initially, a message is subjected to extensive structural regulation through alternative splicing, which is capable of greatly expanding the protein repertoire by generating, in some cases, thousands of functionally distinct isoforms from a single gene locus. Then, RNA packaging into neuronal transport granules and recognition by RNA-binding proteins and/or microRNAs is capable of restricting protein synthesis to selective locations and under specific input conditions. This ability of the post-transcriptional apparatus to expand the informational content of a cell and control the deployment of proteins in both spatial and temporal dimensions is a feature well adapted for the extreme morphological properties of neural cells. In this review, we describe recent advances in understanding how post-transcriptional regulatory mechanisms refine the proteomic complexity required for the assembly of intricate and specific neural networks.
Figures



References
-
- Antar LN, Li C, Zhang H, Carroll RC, Bassell GJ. Local functions for FMRP in axon growth cone motility and activity-dependent regulation of filopodia and spine synapses. Mol Cell Neurosci. 2006;32:37–48. - PubMed
-
- Bartel DP, Chen CZ. Micromanagers of gene expression: The potentially widespread influence of metazoan microRNAs. Nat Rev Genet. 2004;5:396–400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
