Deletion of proapoptotic Puma selectively protects hematopoietic stem and progenitor cells against high-dose radiation
- PMID: 20360471
- PMCID: PMC2890171
- DOI: 10.1182/blood-2009-10-248872
Deletion of proapoptotic Puma selectively protects hematopoietic stem and progenitor cells against high-dose radiation
Abstract
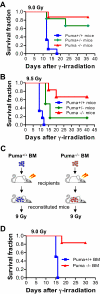
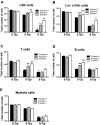
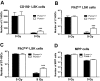
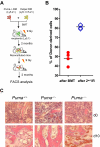
Bone marrow injury is a major adverse side effect of radiation and chemotherapy. Attempts to limit such damage are warranted, but their success requires a better understanding of how radiation and anticancer drugs harm the bone marrow. Here, we report one pivotal role of the BH3-only protein Puma in the radiosensitivity of hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs). Puma deficiency in mice confers resistance to high-dose radiation in a hematopoietic cell-autonomous manner. Unexpectedly, loss of one Puma allele is sufficient to confer mice radioresistance. Interestingly, null mutation in Puma protects both primitive and differentiated hematopoietic cells from damage caused by low-dose radiation but selectively protects HSCs and HPCs against high-dose radiation, thereby accelerating hematopoietic regeneration. Consistent with these findings, Puma is required for radiation-induced apoptosis in HSCs and HPCs, and Puma is selectively induced by irradiation in primitive hematopoietic cells, and this induction is impaired in Puma-heterozygous cells. Together, our data indicate that selective targeting of p53 downstream apoptotic targets may represent a novel strategy to protecting HSCs and HPCs in patients undergoing intensive cancer radiotherapy and chemotherapy.
Figures

Similar articles
-
Deletion of Puma protects hematopoietic stem cells and confers long-term survival in response to high-dose gamma-irradiation.Blood. 2010 Apr 29;115(17):3472-80. doi: 10.1182/blood-2009-10-248278. Epub 2010 Feb 22. Blood. 2010. PMID: 20177048 Free PMC article.
-
Deletion of Irf5 protects hematopoietic stem cells from DNA damage-induced apoptosis and suppresses γ-irradiation-induced thymic lymphomagenesis.Oncogene. 2014 Jun 19;33(25):3288-97. doi: 10.1038/onc.2013.295. Epub 2013 Aug 5. Oncogene. 2014. PMID: 23912454
-
Interleukin-33 regulates hematopoietic stem cell regeneration after radiation injury.Stem Cell Res Ther. 2019 Apr 18;10(1):123. doi: 10.1186/s13287-019-1221-1. Stem Cell Res Ther. 2019. PMID: 30999922 Free PMC article.
-
Regulation of hematopoietic stem cell integrity through p53 and its related factors.Ann N Y Acad Sci. 2016 Apr;1370(1):45-54. doi: 10.1111/nyas.12986. Epub 2015 Dec 22. Ann N Y Acad Sci. 2016. PMID: 26695737 Review.
-
No PUMA, no death: implications for p53-dependent apoptosis.Cancer Cell. 2003 Oct;4(4):248-9. doi: 10.1016/s1535-6108(03)00249-6. Cancer Cell. 2003. PMID: 14585351 Review.
Cited by
-
Fluacrypyrim Protects Hematopoietic Stem and Progenitor Cells against Irradiation via Apoptosis Prevention.Molecules. 2024 Feb 9;29(4):816. doi: 10.3390/molecules29040816. Molecules. 2024. PMID: 38398568 Free PMC article.
-
Role of p53 in regulating tissue response to radiation by mechanisms independent of apoptosis.Transl Cancer Res. 2013 Oct;2(5):412-421. Transl Cancer Res. 2013. PMID: 24466508 Free PMC article.
-
Uncoupling p53 functions in radiation-induced intestinal damage via PUMA and p21.Mol Cancer Res. 2011 May;9(5):616-25. doi: 10.1158/1541-7786.MCR-11-0052. Epub 2011 Mar 30. Mol Cancer Res. 2011. PMID: 21450905 Free PMC article.
-
Whole body proton irradiation causes acute damage to bone marrow hematopoietic progenitor and stem cells in mice.Int J Radiat Biol. 2017 Dec;93(12):1312-1320. doi: 10.1080/09553002.2017.1356941. Epub 2017 Aug 7. Int J Radiat Biol. 2017. PMID: 28782442 Free PMC article.
-
Targeting survivin sensitizes cervical cancer cells to radiation treatment.Bioengineered. 2020 Dec;11(1):130-140. doi: 10.1080/21655979.2020.1717297. Bioengineered. 2020. PMID: 31959045 Free PMC article.
References
-
- Werner-Wasik M, Yu X, Marks LB, Schultheiss TE. Normal-tissue toxicities of thoracic radiation therapy: esophagus, lung, and spinal cord as organs at risk. Hematol Oncol Clin North Am. 2004;18(1):131–160. x–xi. - PubMed
-
- Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293(5528):293–297. - PubMed
-
- Lotem J, Sachs L. Hematopoietic cells from mice deficient in wild-type p53 are more resistant to induction of apoptosis by some agents. Blood. 1993;82(4):1092–1096. - PubMed
-
- Wlodarski P, Wasik M, Ratajczak MZ, et al. Role of p53 in hematopoietic recovery after cytotoxic treatment. Blood. 1998;91(8):2998–3006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

