Pericentrosomal targeting of Rab6 secretory vesicles by Bicaudal-D-related protein 1 (BICDR-1) regulates neuritogenesis
- PMID: 20360680
- PMCID: PMC2876961
- DOI: 10.1038/emboj.2010.51
Pericentrosomal targeting of Rab6 secretory vesicles by Bicaudal-D-related protein 1 (BICDR-1) regulates neuritogenesis
Abstract
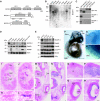
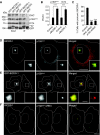
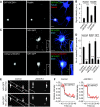
Membrane and secretory trafficking are essential for proper neuronal development. However, the molecular mechanisms that organize secretory trafficking are poorly understood. Here, we identify Bicaudal-D-related protein 1 (BICDR-1) as an effector of the small GTPase Rab6 and key component of the molecular machinery that controls secretory vesicle transport in developing neurons. BICDR-1 interacts with kinesin motor Kif1C, the dynein/dynactin retrograde motor complex, regulates the pericentrosomal localization of Rab6-positive secretory vesicles and is required for neural development in zebrafish. BICDR-1 expression is high during early neuronal development and strongly declines during neurite outgrowth. In young neurons, BICDR-1 accumulates Rab6 secretory vesicles around the centrosome, restricts anterograde secretory transport and inhibits neuritogenesis. Later during development, BICDR-1 expression is strongly reduced, which permits anterograde secretory transport required for neurite outgrowth. These results indicate an important role for BICDR-1 as temporal regulator of secretory trafficking during the early phase of neuronal differentiation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Comment in
-
The more, the better: the BICD family gets bigger.EMBO J. 2010 May 19;29(10):1625-6. doi: 10.1038/emboj.2010.78. EMBO J. 2010. PMID: 20485297 Free PMC article. No abstract available.
Similar articles
-
Bicaudal d family adaptor proteins control the velocity of Dynein-based movements.Cell Rep. 2014 Sep 11;8(5):1248-56. doi: 10.1016/j.celrep.2014.07.052. Epub 2014 Aug 28. Cell Rep. 2014. PMID: 25176647
-
A role for the Rab6B Bicaudal-D1 interaction in retrograde transport in neuronal cells.Exp Cell Res. 2007 Oct 1;313(16):3408-20. doi: 10.1016/j.yexcr.2007.05.032. Epub 2007 Jul 17. Exp Cell Res. 2007. PMID: 17707369
-
Distinct sets of Rab6 effectors contribute to ZW10--and COG-dependent Golgi homeostasis.Traffic. 2014 Jun;15(6):630-47. doi: 10.1111/tra.12167. Epub 2014 Apr 11. Traffic. 2014. PMID: 24575842 Free PMC article.
-
Neuronal Roles of the Bicaudal D Family of Motor Adaptors.Vitam Horm. 2017;104:133-152. doi: 10.1016/bs.vh.2016.11.005. Epub 2016 Dec 29. Vitam Horm. 2017. PMID: 28215293 Review.
-
Rab-mediated trafficking role in neurite formation.J Neurochem. 2014 Apr;129(2):240-8. doi: 10.1111/jnc.12676. Epub 2014 Mar 10. J Neurochem. 2014. PMID: 24517494 Review.
Cited by
-
Structure and Mechanics of Dynein Motors.Annu Rev Biophys. 2021 May 6;50:549-574. doi: 10.1146/annurev-biophys-111020-101511. Annu Rev Biophys. 2021. PMID: 33957056 Free PMC article. Review.
-
Are Rab proteins the link between Golgi organization and membrane trafficking?Cell Mol Life Sci. 2012 Dec;69(24):4093-106. doi: 10.1007/s00018-012-1021-6. Epub 2012 May 13. Cell Mol Life Sci. 2012. PMID: 22581368 Free PMC article. Review.
-
Dynein activator Hook1 is required for trafficking of BDNF-signaling endosomes in neurons.J Cell Biol. 2019 Jan 7;218(1):220-233. doi: 10.1083/jcb.201805016. Epub 2018 Oct 29. J Cell Biol. 2019. PMID: 30373907 Free PMC article.
-
Exome sequencing links corticospinal motor neuron disease to common neurodegenerative disorders.Science. 2014 Jan 31;343(6170):506-511. doi: 10.1126/science.1247363. Science. 2014. PMID: 24482476 Free PMC article.
-
moSCminer: a cell subtype classification framework based on the attention neural network integrating the single-cell multi-omics dataset on the cloud.PeerJ. 2024 Feb 26;12:e17006. doi: 10.7717/peerj.17006. eCollection 2024. PeerJ. 2024. PMID: 38426141 Free PMC article.
References
-
- Babb SG, Kotradi SM, Shah B, Chiappini-Williamson C, Bell LN, Schmeiser G, Chen E, Liu Q, Marrs JA (2005) Zebrafish R-cadherin (Cdh4) controls visual system development and differentiation. Dev Dyn 233: 930–945 - PubMed
-
- Bonifacino JS, Glick BS (2004) The mechanisms of vesicle budding and fusion. Cell 116: 153–166 - PubMed
-
- Bradke F, Dotti CG (2000) Differentiated neurons retain the capacity to generate axons from dendrites. Curr Biol 10: 1467–1470 - PubMed
-
- Bullock SL, Ish-Horowicz D (2001) Conserved signals and machinery for RNA transport in Drosophila oogenesis and embryogenesis. Nature 414: 611–616 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

