Viral apoptosis is induced by IRF-3-mediated activation of Bax
- PMID: 20360684
- PMCID: PMC2876960
- DOI: 10.1038/emboj.2010.50
Viral apoptosis is induced by IRF-3-mediated activation of Bax
Abstract
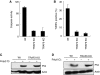
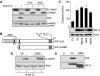
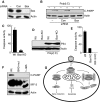
Upon infection with many RNA viruses, the cytoplasmic retinoic acid inducible gene-I (RIG-I) pathway activates the latent transcription factor IRF-3, causing its nuclear translocation and the induction of many antiviral genes, including those encoding interferons. Here, we report a novel and distinct activity of IRF-3, in virus-infected cells, that induces apoptosis. Using genetically defective mouse and human cell lines, we demonstrated that, although both pathways required the presence of RIG-I, IPS1, TRAF3 and TBK1, only the apoptotic pathway required the presence of TRAF2 and TRAF6 in addition. More importantly, transcriptionally inactive IRF-3 mutants, such as the one missing its DNA-binding domain, could efficiently mediate apoptosis. Apoptosis was triggered by the direct interaction of IRF-3, through a newly identified BH3 domain, with the pro-apoptotic protein Bax, their co-translocation to the mitochondria and the resulting activation of the mitochondrial apoptotic pathway. Thus, IRF-3 is a dual-action cytoplasmic protein that, upon activation, translocates to the nucleus or to the mitochondrion and triggers two complementary antiviral responses of the infected cell.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

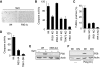




Comment in
-
IRF-3 partners Bax in a viral-induced dance macabre.EMBO J. 2010 May 19;29(10):1627-8. doi: 10.1038/emboj.2010.79. EMBO J. 2010. PMID: 20485298 Free PMC article. No abstract available.
References
-
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA (2001) Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413: 732–738 - PubMed
-
- Barber GN (2001) Host defense, viruses and apoptosis. Cell Death Differ 8: 113–126 - PubMed
-
- Doyle S, Vaidya S, O'Connell R, Dadgostar H, Dempsey P, Wu T, Rao G, Sun R, Haberland M, Modlin R, Cheng G (2002) IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 17: 251–263 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

