AIF promotes chromatinolysis and caspase-independent programmed necrosis by interacting with histone H2AX
- PMID: 20360685
- PMCID: PMC2876946
- DOI: 10.1038/emboj.2010.43
AIF promotes chromatinolysis and caspase-independent programmed necrosis by interacting with histone H2AX
Abstract
Programmed necrosis induced by DNA alkylating agents, such as MNNG, is a caspase-independent mode of cell death mediated by apoptosis-inducing factor (AIF). After poly(ADP-ribose) polymerase 1, calpain, and Bax activation, AIF moves from the mitochondria to the nucleus where it induces chromatinolysis and cell death. The mechanisms underlying the nuclear action of AIF are, however, largely unknown. We show here that, through its C-terminal proline-rich binding domain (PBD, residues 543-559), AIF associates in the nucleus with histone H2AX. This interaction regulates chromatinolysis and programmed necrosis by generating an active DNA-degrading complex with cyclophilin A (CypA). Deletion or directed mutagenesis in the AIF C-terminal PBD abolishes AIF/H2AX interaction and AIF-mediated chromatinolysis. H2AX genetic ablation or CypA downregulation confers resistance to programmed necrosis. AIF fails to induce chromatinolysis in H2AX or CypA-deficient nuclei. We also establish that H2AX is phosphorylated at Ser139 after MNNG treatment and that this phosphorylation is critical for caspase-independent programmed necrosis. Overall, our data shed new light in the mechanisms regulating programmed necrosis, elucidate a key nuclear partner of AIF, and uncover an AIF apoptogenic motif.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
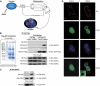

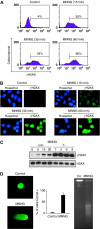
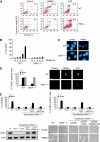

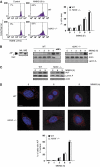
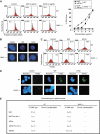
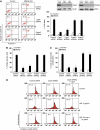
References
-
- Artus C, Maquarre E, Moubarak RS, Delettre C, Jasmin C, Susin SA, Robert-Lezenes J (2006) CD44 ligation induces caspase-independent cell death via a novel calpain/AIF pathway in human erythroleukemia cells. Oncogene 25: 5741–5751 - PubMed
-
- Banath JP, Olive PL (2003) Expression of phosphorylated histone H2AX as a surrogate of cell killing by drugs that create DNA double-strand breaks. Cancer Res 63: 4347–4350 - PubMed
-
- Cande C, Vahsen N, Kouranti I, Schmitt E, Daugas E, Spahr C, Luban J, Kroemer RT, Giordanetto F, Garrido C, Penninger JM, Kroemer G (2004) AIF and cyclophilin A cooperate in apoptosis-associated chromatinolysis. Oncogene 23: 1514–1521 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

