Phenotypic profiling of the human genome by time-lapse microscopy reveals cell division genes
- PMID: 20360735
- PMCID: PMC3108885
- DOI: 10.1038/nature08869
Phenotypic profiling of the human genome by time-lapse microscopy reveals cell division genes
Abstract
Despite our rapidly growing knowledge about the human genome, we do not know all of the genes required for some of the most basic functions of life. To start to fill this gap we developed a high-throughput phenotypic screening platform combining potent gene silencing by RNA interference, time-lapse microscopy and computational image processing. We carried out a genome-wide phenotypic profiling of each of the approximately 21,000 human protein-coding genes by two-day live imaging of fluorescently labelled chromosomes. Phenotypes were scored quantitatively by computational image processing, which allowed us to identify hundreds of human genes involved in diverse biological functions including cell division, migration and survival. As part of the Mitocheck consortium, this study provides an in-depth analysis of cell division phenotypes and makes the entire high-content data set available as a resource to the community.
Figures


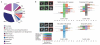
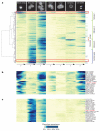
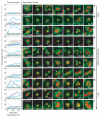
Comment in
-
Cell biology forum: Genome-wide view of mitosis.Nature. 2010 Apr 1;464(7289):684-5. doi: 10.1038/464684a. Nature. 2010. PMID: 20360724 No abstract available.
-
Discovering Mozart.Nat Methods. 2010 Jun;7(6):421. doi: 10.1038/nmeth0610-421. Nat Methods. 2010. PMID: 20521367
References
-
- Neumann B, et al. High-throughput RNAi screening by time-lapse imaging of live human cells. Nature Methods. 2006;3:385–390. - PubMed
-
- Walter T, et al. Automatic identification and clustering of chromosome phenotypes in a genome wide RNAi screen by time-lapse imaging. J. Struct. Biol. (in the press) - PubMed
-
- Boland MV, Markey MK, Murphy RF. Automated recognition of patterns characteristic of subcellular structures in fluorescence microscopy images. Cytometry. 1998;33:366–375. - PubMed
-
- Glory E, Murphy RF. Automated subcellular location determination and high-throughput microscopy. Dev. Cell. 2007;12:7–16. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

