Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia
- PMID: 20360742
- PMCID: PMC2864584
- DOI: 10.1038/nature08855
Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia
Abstract
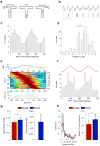
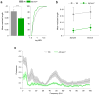
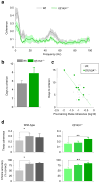
Abnormalities in functional connectivity between brain areas have been postulated as an important pathophysiological mechanism underlying schizophrenia. In particular, macroscopic measurements of brain activity in patients suggest that functional connectivity between the frontal and temporal lobes may be altered. However, it remains unclear whether such dysconnectivity relates to the aetiology of the illness, and how it is manifested in the activity of neural circuits. Because schizophrenia has a strong genetic component, animal models of genetic risk factors are likely to aid our understanding of the pathogenesis and pathophysiology of the disease. Here we study Df(16)A(+/-) mice, which model a microdeletion on human chromosome 22 (22q11.2) that constitutes one of the largest known genetic risk factors for schizophrenia. To examine functional connectivity in these mice, we measured the synchronization of neural activity between the hippocampus and the prefrontal cortex during the performance of a task requiring working memory, which is one of the cognitive functions disrupted in the disease. In wild-type mice, hippocampal-prefrontal synchrony increased during working memory performance, consistent with previous reports in rats. Df(16)A(+/-) mice, which are impaired in the acquisition of the task, showed drastically reduced synchrony, measured both by phase-locking of prefrontal cells to hippocampal theta oscillations and by coherence of prefrontal and hippocampal local field potentials. Furthermore, the magnitude of hippocampal-prefrontal coherence at the onset of training could be used to predict the time it took the Df(16)A(+/-) mice to learn the task and increased more slowly during task acquisition. These data suggest how the deficits in functional connectivity observed in patients with schizophrenia may be realized at the single-neuron level. Our findings further suggest that impaired long-range synchrony of neural activity is one consequence of the 22q11.2 deletion and may be a fundamental component of the pathophysiology underlying schizophrenia.
Figures

References
-
- Wernicke C. Grundrisse der Psychiatrie. Thieme; 1906.
-
- Meyer-Lindenberg AS, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–386. - PubMed
-
- Lawrie SM, et al. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry. 2002;51:1008–1011. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

