Tgf-Beta inhibition restores terminal osteoblast differentiation to suppress myeloma growth
- PMID: 20360846
- PMCID: PMC2845613
- DOI: 10.1371/journal.pone.0009870
Tgf-Beta inhibition restores terminal osteoblast differentiation to suppress myeloma growth
Abstract
Background: Multiple myeloma (MM) expands almost exclusively in the bone marrow and generates devastating bone lesions, in which bone formation is impaired and osteoclastic bone resorption is enhanced. TGF-beta, a potent inhibitor of terminal osteoblast (OB) differentiation, is abundantly deposited in the bone matrix, and released and activated by the enhanced bone resorption in MM. The present study was therefore undertaken to clarify the role of TGF-beta and its inhibition in bone formation and tumor growth in MM.
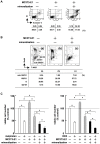
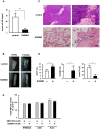
Methodology/principal findings: TGF-beta suppressed OB differentiation from bone marrow stromal cells and MC3T3-E1 preosteoblastic cells, and also inhibited adipogenesis from C3H10T1/2 immature mesenchymal cells, suggesting differentiation arrest by TGF-beta. Inhibitors for a TGF-beta type I receptor kinase, SB431542 and Ki26894, potently enhanced OB differentiation from bone marrow stromal cells as well as MC3T3-E1 cells. The TGF-beta inhibition was able to restore OB differentiation suppressed by MM cell conditioned medium as well as bone marrow plasma from MM patients. Interestingly, TGF-beta inhibition expedited OB differentiation in parallel with suppression of MM cell growth. The anti-MM activity was elaborated exclusively by terminally differentiated OBs, which potentiated the cytotoxic effects of melphalan and dexamethasone on MM cells. Furthermore, TGF-beta inhibition was able to suppress MM cell growth within the bone marrow while preventing bone destruction in MM-bearing animal models.
Conclusions/significance: The present study demonstrates that TGF-beta inhibition releases stromal cells from their differentiation arrest by MM and facilitates the formation of terminally differentiated OBs, and that terminally differentiated OBs inhibit MM cell growth and survival and enhance the susceptibility of MM cells to anti-MM agents to overcome the drug resistance mediated by stromal cells. Therefore, TGF-beta appears to be an important therapeutic target in MM bone lesions.
Conflict of interest statement
Figures





References
-
- Abe M, Hiura K, Wilde J, Moriyama K, Hashimoto T, et al. Role for macrophage inflammatory protein (MIP)-1alpha and MIP-1beta in the development of osteolytic lesions in multiple myeloma. Blood. 2002;100:2195–2202. - PubMed
-
- Hashimoto T, Abe M, Oshima T, Shibata H, Ozaki S, et al. Ability of myeloma cells to secrete macrophage inflammatory protein (MIP)-1alpha and MIP-1beta correlates with lytic bone lesions in patients with multiple myeloma. Br J Haematol. 2004;125:38–41. - PubMed
-
- Abe M, Hiura K, Ozaki S, Kido S, Matsumoto T. Vicious cycle between myeloma cell binding to bone marrow stromal cells via VLA-4-VCAM-1 adhesion and macrophage inflammatory protein-1alpha and MIP-1beta production. J Bone Miner Metab. 2009;27:16–23. - PubMed
-
- Oshima T, Abe M, Asano J, Hara T, Kitazoe K, et al. Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood. 2005;106:3160–3165. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

