An expanded self-antigen peptidome is carried by the human lymph as compared to the plasma
- PMID: 20360855
- PMCID: PMC2845622
- DOI: 10.1371/journal.pone.0009863
An expanded self-antigen peptidome is carried by the human lymph as compared to the plasma
Abstract
Background: The pre-nodal afferent lymph is the fluid which directly derives from the extracellular milieu from every parenchymal organ and, as it continues to circulate between the cells, it collects products deriving from the organ metabolism/catabolism. A comprehensive qualitative and quantitative investigation of the self-antigenic repertoire transported by the human lymph is still missing.
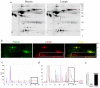
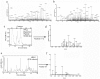
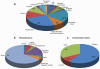
Methodology/principal findings: A major difference between lymph and plasma could be visualized by FPLC and 2D gel in the amount of low molecular weight products corresponding to peptide fragments. Naturally processed peptides in normal pre-nodal human lymph were then fractionated by HPLC and characterized by multidimensional mass spectrometry. Analysis of more then 300 sequences identified self-peptides derived from both intracellular and extracellular proteins revealing the variety of catabolic products transported by human lymph. Quantitative analysis established that at least some of these peptides are present in the circulating lymph in nanomolar concentration.
Conclusions/significance: The peptidome, generated by physiological tissue catabolism and transported by the pre-nodal lymph, is in addition to the self-peptidome generated in endosomal compartment. Unlike self antigen processed by local or nodal APC, which mostly produce epitopes constrained by the endosomal processing activity, self antigens present in the lymph could derived from a wider variety of processing pathways; including caspases, involved in cellular apoptosis, and ADAM and other metalloproteinases involved in surface receptor editing, cytokines processing and matrix remodeling. Altogether, expanding the tissue-specific self-repertoire available for the maintenance of immunological tolerance.
Conflict of interest statement
Figures



References
-
- Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev. 2007;219:143–156. - PubMed
-
- Pabst O, Bernhardt G, Forster R. The impact of cell-bound antigen transport on mucosal tolerance induction. J Leukoc Biol. 2007;82:795–800. - PubMed
-
- Jahnsen, FL, Strickland DH, Thomas JA, Tobagus IT, Napoli S, et al. Accelerated antigen sampling and transport by airway mucosal dendritic cells following inhalation of a bacterial stimulus. J Immunol. 2006;177:5861–5867. - PubMed
-
- Hunger RE, Yawalkar N, Braathen LR, Brand CU. CD1a-positive dendritic cells transport the antigen DNCB intracellularly from the skin to the regional lymph nodes in the induction phase of allergic contact dermatitis. Arch Dermatol Res. 2001;293:420–426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

