KGF Promotes Paracrine Activation of the SCF/c-KIT Axis from Human Keratinocytes to Melanoma Cells
- PMID: 20360932
- PMCID: PMC2847315
- DOI: 10.1593/tlo.09196
KGF Promotes Paracrine Activation of the SCF/c-KIT Axis from Human Keratinocytes to Melanoma Cells
Abstract
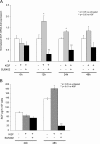
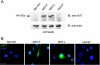
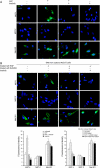
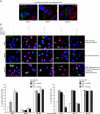
The paracrine networks of the human melanoma microenvironment are able to influence tumor growth and progression. Among the paracrine growth factors involved in skin homeostasis, the KGF/FGF7 secreted by dermal fibroblasts promotes the epidermal proliferation and differentiation as well as the release from keratinocytes of other paracrine mediators. To evaluate the possible role played by KGF in affecting the behavior of different subtypes of melanoma carrying activating mutations or overexpression of the SCF receptor c-KIT, we used human melanoma cell lines, characterized by different expression levels of c-KIT and opposing responsivity to SCF, and HaCaT keratinocytes. Quantitative real-time reverse transcription-polymerase chain reaction assay and ELISA test on KGF-treated keratinocytes showed enhanced expression and secretion of SCF in response to KGF and dependent on functional KGF receptor. Immunofluorescence microscopy and biochemical analysis showed, in one of the selected melanoma cell models, SCF-dependent c-KIT activation induced by stimulation with the culture supernatants collected from KGF-treated keratinocytes. In keratinocyte-melanoma cocultures stained for the Ki67 proliferation marker, incubation with KGF induced enhanced growth not only of the keratinocytes but also of the melanoma cells, which could be blocked by the c-KIT inhibitor imatinib, demonstrating the establishment of a KGF-induced paracrine signaling network owing to the coexpression of biologically active SCF released from keratinocytes and functional c-KIT on melanoma cells.
Figures

Similar articles
-
Cholesteatoma-associated fibroblasts modulate epithelial growth and differentiation through KGF/FGF7 secretion.Histochem Cell Biol. 2012 Aug;138(2):251-69. doi: 10.1007/s00418-012-0947-y. Epub 2012 Apr 6. Histochem Cell Biol. 2012. PMID: 22481617 Free PMC article.
-
Effect of tumour-cell-derived or recombinant keratinocyte growth factor (KGF) on proliferation and radioresponse of human epithelial tumour cells (HNSCC) and normal keratinocytes in vitro.Radiat Environ Biophys. 2010 May;49(2):261-70. doi: 10.1007/s00411-010-0271-7. Epub 2010 Mar 7. Radiat Environ Biophys. 2010. PMID: 20213138 Free PMC article.
-
Keratinocyte growth factor receptor ligands induce transforming growth factor alpha expression and activate the epidermal growth factor receptor signaling pathway in cultured epidermal keratinocytes.Cell Growth Differ. 1994 Dec;5(12):1283-92. Cell Growth Differ. 1994. PMID: 7535082
-
Keratinocyte Growth Factor-Based Strategies for Wound Re-Epithelialization.Tissue Eng Part B Rev. 2022 Jun;28(3):665-676. doi: 10.1089/ten.TEB.2021.0030. Epub 2021 Oct 18. Tissue Eng Part B Rev. 2022. PMID: 34238035 Review.
-
An overview on keratinocyte growth factor: from the molecular properties to clinical applications.Protein Pept Lett. 2014 Mar;21(3):306-17. doi: 10.2174/09298665113206660115. Protein Pept Lett. 2014. PMID: 24188496 Review.
Cited by
-
Possible Role of Interleukin-31/33 Axis in Imatinib Mesylate-Associated Skin Toxicity.Turk J Haematol. 2015 Jun;32(2):168-71. doi: 10.4274/tjh.2014.0021. Turk J Haematol. 2015. PMID: 26316486 Free PMC article.
-
MicroRNA-155 mediates multiple gene regulations pertinent to the role of human adipose-derived mesenchymal stem cells in skin regeneration.Front Bioeng Biotechnol. 2024 Mar 18;12:1328504. doi: 10.3389/fbioe.2024.1328504. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38562669 Free PMC article.
-
Melanoma stimulates the proteolytic activity of HaCaT keratinocytes.Cell Commun Signal. 2022 Sep 19;20(1):146. doi: 10.1186/s12964-022-00961-w. Cell Commun Signal. 2022. PMID: 36123693 Free PMC article.
-
Insights into Differentiation of Melanocytes from Human Stem Cells and Their Relevance for Melanoma Treatment.Cancers (Basel). 2020 Sep 3;12(9):2508. doi: 10.3390/cancers12092508. Cancers (Basel). 2020. PMID: 32899370 Free PMC article. Review.
-
Unremitting cell proliferation in the secretory phase of eutopic endometriosis: involvement of pAkt and pGSK3β.Reprod Sci. 2015 Apr;22(4):502-10. doi: 10.1177/1933719114549843. Epub 2014 Sep 6. Reprod Sci. 2015. PMID: 25194152 Free PMC article.
References
-
- Chin L, Garraway LA, Fisher DE. Malignant melanoma: genetics and therapeutics in the genomic era. Genes Dev. 2006;20:2149–2182. - PubMed
-
- Zaidi MR, Day CP, Merlino G. From UV to metastases: modeling melanoma initiation and progression in the mouse. J Invest Dermatol. 2008;128:2381–2391. - PubMed
-
- Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Bröcker EB, LeBoit PE, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. - PubMed
-
- Goel VK, Lazar AJ, Warneke CL, Redston MS, Haluska FG. Examination of mutations in BRAF, NRAS, and PTEN in primary cutaneous melanoma. J Invest Dermatol. 2006;126:154–160. - PubMed
-
- Muthusamy V, Hobbs C, Nogueira C, Cordon-Cardo C, McKee PH, Chin L, Bosenberg MW. Amplification of CDK4 and MDM2 in malignant melanoma. Genes Chromosomes Cancer. 2006;45:447–454. - PubMed
LinkOut - more resources
Full Text Sources
