Expression of Long-chain Fatty Acyl-CoA Synthetase 4 in Breast and Prostate Cancers Is Associated with Sex Steroid Hormone Receptor Negativity
- PMID: 20360933
- PMCID: PMC2847316
- DOI: 10.1593/tlo.09202
Expression of Long-chain Fatty Acyl-CoA Synthetase 4 in Breast and Prostate Cancers Is Associated with Sex Steroid Hormone Receptor Negativity
Abstract
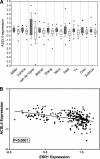
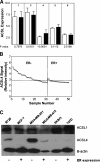
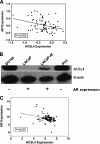
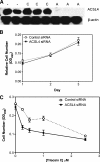
Previous studies have shown that key enzymes involved in lipid metabolic pathways are differentially expressed in normal compared with tumor tissues. However, the precise role played by dysregulated expression of lipid metabolic enzymes and altered lipid homeostasis in carcinogenesis remains to be established. Fatty acid synthase is overexpressed in a variety of cancers, including breast and prostate. The purpose of the present study was to examine the expression patterns of additional lipid metabolic enzymes in human breast and prostate cancers. This was accomplished by analysis of published expression databases, with confirmation by immunoblot assays. Our results indicate that the fatty acid-activating enzyme, long-chain fatty acyl-CoA synthetase 4 (ACSL4), is differentially expressed in human breast cancer as a function of estrogen receptor alpha (ER) status. In 10 separate studies, ACSL4 messenger RNA (mRNA) was overexpressed in ER-negative breast tumors. Of 50 breast cancer cell lines examined, 17 (89%) of 19 ER-positive lines were negative for ACSL4 mRNA expression and 20 (65%) of 31 ER-negative lines expressed ACSL4 mRNA. The inverse relationship between ER expression and ACSL4 expression was also observed for androgen receptor status in both breast and prostate cancers. Furthermore, loss of steroid hormone sensitivity, such as that observed in Raf1-transfected MCF-7 cells and LNCaP-AI cells, was associated with induction of ACSL4 expression. Ablation of ACSL4 expression inMDA-MB-231 breast cancer cells had no effect on cell proliferation; however, sensitivity to the cytotoxic effects of triacsin C was increased three-fold in the cells lacking ACSL4.
Figures

Similar articles
-
Long chain fatty Acyl-CoA synthetase 4 is a biomarker for and mediator of hormone resistance in human breast cancer.PLoS One. 2013 Oct 14;8(10):e77060. doi: 10.1371/journal.pone.0077060. eCollection 2013. PLoS One. 2013. PMID: 24155918 Free PMC article.
-
Association of long-chain acyl-coenzyme A synthetase 5 expression in human breast cancer by estrogen receptor status and its clinical significance.Oncol Rep. 2017 Jun;37(6):3253-3260. doi: 10.3892/or.2017.5610. Epub 2017 Apr 28. Oncol Rep. 2017. PMID: 28498416
-
Acyl-CoA synthetase-4 is implicated in drug resistance in breast cancer cell lines involving the regulation of energy-dependent transporter expression.Biochem Pharmacol. 2019 Jan;159:52-63. doi: 10.1016/j.bcp.2018.11.005. Epub 2018 Nov 9. Biochem Pharmacol. 2019. PMID: 30414939
-
ACSL4 as a Potential Target and Biomarker for Anticancer: From Molecular Mechanisms to Clinical Therapeutics.Front Pharmacol. 2022 Jul 13;13:949863. doi: 10.3389/fphar.2022.949863. eCollection 2022. Front Pharmacol. 2022. PMID: 35910359 Free PMC article. Review.
-
ACSL4: biomarker, mediator and target in quadruple negative breast cancer.Oncotarget. 2023 Jun 12;14:563-575. doi: 10.18632/oncotarget.28453. Oncotarget. 2023. PMID: 37306503 Free PMC article. Review.
Cited by
-
Identification of MiR-211-5p as a tumor suppressor by targeting ACSL4 in Hepatocellular Carcinoma.J Transl Med. 2020 Aug 28;18(1):326. doi: 10.1186/s12967-020-02494-7. J Transl Med. 2020. PMID: 32859232 Free PMC article.
-
Reprogramming of Fatty Acid Metabolism in Gynaecological Cancers: Is There a Role for Oestradiol?Metabolites. 2022 Apr 14;12(4):350. doi: 10.3390/metabo12040350. Metabolites. 2022. PMID: 35448537 Free PMC article. Review.
-
Acyl-CoA metabolism and partitioning.Annu Rev Nutr. 2014;34:1-30. doi: 10.1146/annurev-nutr-071813-105541. Epub 2014 Apr 10. Annu Rev Nutr. 2014. PMID: 24819326 Free PMC article. Review.
-
Fatty acid metabolism in breast cancer subtypes.Oncotarget. 2017 Apr 25;8(17):29487-29500. doi: 10.18632/oncotarget.15494. Oncotarget. 2017. PMID: 28412757 Free PMC article. Review.
-
Systematic Analysis of Gene Expression Alterations and Clinical Outcomes for Long-Chain Acyl-Coenzyme A Synthetase Family in Cancer.PLoS One. 2016 May 12;11(5):e0155660. doi: 10.1371/journal.pone.0155660. eCollection 2016. PLoS One. 2016. PMID: 27171439 Free PMC article.
References
-
- Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7(10)(2):63–777. - PubMed
-
- Brunet J, Vazquez-Martin A, Colomer R, Graña-Suarez B, Martin-Castillo B, Menendez JA. BRCA1 and acetyl-CoA carboxylase: the metabolic syndrome of breast cancer. Mol Carcinog. 2008;47(2):157–163. - PubMed
-
- Black PN, Fargeman NJ, DiRusso CC. Long-chain acyl-CoA-dependent regulation of gene expression in bacteria, yeast and mammals. J Nutr. 2000;130(2):305s–309s. - PubMed
-
- Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res. 2004;43:134–176. - PubMed
-
- Cao Y, Traer E, Zimmerman GA, McIntyre TM, Prescott SM. Cloning, expression, and chromosomal localization of human long-chain fatty acid-CoA ligase 4 (FACL4) Genomics. 1998;49:327–330. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
