Pigment epithelium-derived factor stimulates tumor macrophage recruitment and is downregulated by the prostate tumor microenvironment
- PMID: 20360944
- PMCID: PMC2847741
- DOI: 10.1593/neo.92046
Pigment epithelium-derived factor stimulates tumor macrophage recruitment and is downregulated by the prostate tumor microenvironment
Abstract
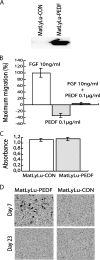
Pigment epithelium-derived factor (PEDF) is a potent inhibitor of angiogenesis but whether it has additional effects on the tumor microenvironment is largely unexplored. We show that overexpression of PEDF in orthotopic MatLyLu rat prostate tumors increased tumor macrophage recruitment. The fraction of macrophages expressing inducible nitric oxide synthase, a marker of cytotoxic M1 macrophages, was increased, suggesting that PEDF could enhance antitumor immunity. In addition, PEDF overexpression reduced vascular growth both in the tumor and in the surrounding normal tissue, slowed tumor growth, and decreased lymph node metastasis. Contrary, extratumoral lymphangiogenesis was increased. PEDF expression is, for reasons unknown, often decreased or lost during prostate tumor progression. When AT-1 rat prostate tumor cells, expressing high levels of PEDF messenger RNA (mRNA) and protein, were injected into the prostate, PEDF is markedly downregulated, suggesting that factors in the microenvironment suppressed its expression. One such factor could be macrophage-derived tumor necrosis factor alpha (TNFalpha). A fraction of the accumulating macrophages expressed TNFalpha, and TNFalpha treatment downregulated the expression of PEDF protein and mRNA in prostate AT-1 tumor cells in vitro and in the rat ventral prostate in vivo. PEDF apparently has multiple effects in prostate tumors: it suppresses angiogenesis and metastasis, but it also causes macrophage accumulation. Accumulating macrophages may inhibit tumor growth, but they may also suppress PEDF and enhance lymph angiogenesis and, in this way, eventually enhance tumor growth.
Figures
Similar articles
-
Positive correlation between PEDF expression levels and macrophage density in the human prostate.Prostate. 2013 Apr;73(5):549-61. doi: 10.1002/pros.22595. Epub 2012 Oct 4. Prostate. 2013. PMID: 23038613 Free PMC article.
-
Vacuolar H+-ATPase is down-regulated by the angiogenesis-inhibitory pigment epithelium-derived factor in metastatic prostate cancer cells.Cell Mol Biol (Noisy-le-grand). 2014 May 25;60(1):45-52. Cell Mol Biol (Noisy-le-grand). 2014. PMID: 24857383
-
Changes in the gene expression profile of A375 human melanoma cells induced by overexpression of multifunctional pigment epithelium-derived factor.Melanoma Res. 2011 Aug;21(4):285-97. doi: 10.1097/CMR.0b013e32834495c3. Melanoma Res. 2011. PMID: 21673604 Free PMC article.
-
PEDF and its roles in physiological and pathological conditions: implication in diabetic and hypoxia-induced angiogenic diseases.Clin Sci (Lond). 2015 Jun;128(11):805-23. doi: 10.1042/CS20130463. Clin Sci (Lond). 2015. PMID: 25881671 Free PMC article. Review.
-
Neuroprotective and antiangiogenic actions of PEDF in the eye: molecular targets and therapeutic potential.Prog Retin Eye Res. 2004 Sep;23(5):561-77. doi: 10.1016/j.preteyeres.2004.05.002. Prog Retin Eye Res. 2004. PMID: 15302351 Review.
Cited by
-
Positive correlation between PEDF expression levels and macrophage density in the human prostate.Prostate. 2013 Apr;73(5):549-61. doi: 10.1002/pros.22595. Epub 2012 Oct 4. Prostate. 2013. PMID: 23038613 Free PMC article.
-
PEDF inhibits pancreatic tumorigenesis by attenuating the fibro-inflammatory reaction.Oncotarget. 2016 May 10;7(19):28218-34. doi: 10.18632/oncotarget.8587. Oncotarget. 2016. PMID: 27058416 Free PMC article.
-
Extratumoral Heme Oxygenase-1 (HO-1) Expressing Macrophages Likely Promote Primary and Metastatic Prostate Tumor Growth.PLoS One. 2016 Jun 9;11(6):e0157280. doi: 10.1371/journal.pone.0157280. eCollection 2016. PLoS One. 2016. PMID: 27280718 Free PMC article.
-
Metformin and Cancer Hallmarks: Molecular Mechanisms in Thyroid, Prostate and Head and Neck Cancer Models.Biomolecules. 2022 Feb 24;12(3):357. doi: 10.3390/biom12030357. Biomolecules. 2022. PMID: 35327549 Free PMC article. Review.
-
PEDF regulates plasticity of a novel lipid-MTOC axis in prostate cancer-associated fibroblasts.J Cell Sci. 2018 Jul 11;131(13):jcs213579. doi: 10.1242/jcs.213579. J Cell Sci. 2018. PMID: 29792311 Free PMC article.
References
-
- Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. - PubMed
-
- Biswas SK, Sica A, Lewis CE. Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J Immunol. 2008;180:2011–2017. - PubMed
-
- Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–355. - PubMed
-
- Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, Bergh A. Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int J Oncol. 2000;17:445–451. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
