Microbial community structure of leaf-cutter ant fungus gardens and refuse dumps
- PMID: 20360970
- PMCID: PMC2847949
- DOI: 10.1371/journal.pone.0009922
Microbial community structure of leaf-cutter ant fungus gardens and refuse dumps
Abstract
Background: Leaf-cutter ants use fresh plant material to grow a mutualistic fungus that serves as the ants' primary food source. Within fungus gardens, various plant compounds are metabolized and transformed into nutrients suitable for ant consumption. This symbiotic association produces a large amount of refuse consisting primarily of partly degraded plant material. A leaf-cutter ant colony is thus divided into two spatially and chemically distinct environments that together represent a plant biomass degradation gradient. Little is known about the microbial community structure in gardens and dumps or variation between lab and field colonies.
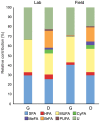
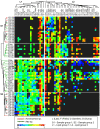
Methodology/principal findings: Using microbial membrane lipid analysis and a variety of community metrics, we assessed and compared the microbiota of fungus gardens and refuse dumps from both laboratory-maintained and field-collected colonies. We found that gardens contained a diverse and consistent community of microbes, dominated by Gram-negative bacteria, particularly gamma-Proteobacteria and Bacteroidetes. These findings were consistent across lab and field gardens, as well as host ant taxa. In contrast, dumps were enriched for Gram-positive and anaerobic bacteria. Broad-scale clustering analyses revealed that community relatedness between samples reflected system component (gardens/dumps) rather than colony source (lab/field). At finer scales samples clustered according to colony source.
Conclusions/significance: Here we report the first comparative analysis of the microbiota from leaf-cutter ant colonies. Our work reveals the presence of two distinct communities: one in the fungus garden and the other in the refuse dump. Though we find some effect of colony source on community structure, our data indicate the presence of consistently associated microbes within gardens and dumps. Substrate composition and system component appear to be the most important factor in structuring the microbial communities. These results thus suggest that resident communities are shaped by the plant degradation gradient created by ant behavior, specifically their fungiculture and waste management.
Conflict of interest statement
Figures



Similar articles
-
Bacteria Contribute to Plant Secondary Compound Degradation in a Generalist Herbivore System.mBio. 2020 Sep 15;11(5):e02146-20. doi: 10.1128/mBio.02146-20. mBio. 2020. PMID: 32934088 Free PMC article.
-
Ultrastructural and microbial analyses of cellulose degradation in leaf-cutter ant colonies.Microbiology (Reading). 2017 Nov;163(11):1578-1589. doi: 10.1099/mic.0.000546. Epub 2017 Oct 16. Microbiology (Reading). 2017. PMID: 29034862
-
Leucoagaricus gongylophorus produces diverse enzymes for the degradation of recalcitrant plant polymers in leaf-cutter ant fungus gardens.Appl Environ Microbiol. 2013 Jun;79(12):3770-8. doi: 10.1128/AEM.03833-12. Epub 2013 Apr 12. Appl Environ Microbiol. 2013. PMID: 23584789 Free PMC article.
-
The Evolutionary Innovation of Nutritional Symbioses in Leaf-Cutter Ants.Insects. 2012 Jan 6;3(1):41-61. doi: 10.3390/insects3010041. Insects. 2012. PMID: 26467948 Free PMC article. Review.
-
The origin of the attine ant-fungus mutualism.Q Rev Biol. 2001 Jun;76(2):169-97. doi: 10.1086/393867. Q Rev Biol. 2001. PMID: 11409051 Review.
Cited by
-
Unique honey bee (Apis mellifera) hive component-based communities as detected by a hybrid of phospholipid fatty-acid and fatty-acid methyl ester analyses.PLoS One. 2015 Apr 7;10(4):e0121697. doi: 10.1371/journal.pone.0121697. eCollection 2015. PLoS One. 2015. PMID: 25849080 Free PMC article.
-
Leaf-cutter ants engineer large nitrous oxide hot spots in tropical forests.Proc Biol Sci. 2019 Jan 16;286(1894):20182504. doi: 10.1098/rspb.2018.2504. Proc Biol Sci. 2019. PMID: 30963857 Free PMC article.
-
Insights on aquatic microbiome of the Indian Sundarbans mangrove areas.PLoS One. 2020 Feb 25;15(2):e0221543. doi: 10.1371/journal.pone.0221543. eCollection 2020. PLoS One. 2020. PMID: 32097429 Free PMC article.
-
Garden microbiomes of Apterostigma dentigerum and Apterostigma pilosum fungus-growing ants (Hymenoptera: Formicidae).J Microbiol. 2019 Oct;57(10):842-851. doi: 10.1007/s12275-019-8639-0. Epub 2019 Aug 3. J Microbiol. 2019. PMID: 31377982
-
Major changes in microbial diversity and community composition across gut sections of a juvenile Panchlora cockroach.PLoS One. 2017 May 18;12(5):e0177189. doi: 10.1371/journal.pone.0177189. eCollection 2017. PLoS One. 2017. PMID: 28545131 Free PMC article.
References
-
- Belt T. London: J. Murray.; 1874. The Naturalist in Nicaragua. p. 403 p.
-
- Weber NA. Fungus-growing ants. Science. 1966;153:587–604. - PubMed
-
- Weber NA. Philadelphia: American Philosophical Society.; 1972. Gardening Ants: The Attines. p. 146 p.
-
- Hölldobler B, Wilson EO. Cambridge, Mass: Belknap Press of Harvard University Press.; 1990. The Ants. p. 752 p.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

