Effects of Detergents on Activity, Thermostability and Aggregation of Two Alkalithermophilic Lipases from Thermosyntropha lipolytica
- PMID: 20361033
- PMCID: PMC2847205
- DOI: 10.2174/1874091X01004010022
Effects of Detergents on Activity, Thermostability and Aggregation of Two Alkalithermophilic Lipases from Thermosyntropha lipolytica
Abstract
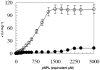
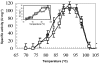
Thermosyntropha lipolytica DSM 11003, an anaerobic thermophilic lipolytic bacterium, produces the two alkalithermophilic lipases, LipA and LipB. Among all tested detergents, the two lipases were mostly affected by SDS when used at concentrations below its critical micelle concentration (CMC). In the absence of SDS, the v(max) of both LipA and LipB were 12.6 U.mg(-1) and 13.3 U.mg(-1) and K(0.5) were 1.8 mM and 1.65 mM, respectively at 96 degrees C and pH(opt) (25 masculineC)of 9.4-9.6. In the presence of 0.2% SDS, the v(max) increased to 105 U.mg(-1) and 112 U.mg(-1), and K(0.5) values decreased to 200 microM and 140 microM for LipA and LipB, respectively. Inhibitory assays of lipases using diisopropyl p-nitrophenylphosphate (E600) with increasing concentration of SDS and Tween 20 strongly suggest that SDS and Tween 20 do bind to the lid domain and/or active site pocket, thus promoting conformational changes that facilitate active site accessibility for the substrate. The two lipases exhibited moderate activation in the presence of nonionic detergents when used below their CMC values. Both lipases were found to exhibit strong tendency to aggregate as observed through gel filtration chromatography and gradient native gel electrophoresis. The addition of 1.0% (w/v) SDS led to disaggregation as the lipases were eluted corresponding to their monomeric mass (based on SDS gel electrophoresis value) and caused a significant decrease in thermostability, suggesting that, enzyme aggregation might be a major contributor to the high thermostability of LipA and LipB.
Keywords: Alkalithermophilic; Lipases; SDS; non ionic- anionic and cationic detergents.; Thermosyntropha lipolytica; aggregation/oligomerization.
Figures



References
-
- Sarda L, Desnuelle P. Action of pancreatic lipase on emulsified esters. Biochim. Biophys. Acta. 1958;30:513–521. - PubMed
-
- Ferrato F, Carriere F, Sarda L, Verger R. A critical reevaluation of the phenomenon of interfacial activation. Methods Enzymol. 1997;286:327–347. - PubMed
-
- Jaeger KE, Reetz MT. Microbial lipases form versatile tools for biotechnology. Trends Biotechnol. 1998;16:396–403. - PubMed
-
- Balcão V, Paiva M, Malcata FX. Bioreactors with immobilized lipases: State of the art. Enzyme Microb. Technol. 1996;18:392–416. - PubMed
-
- Bell PJL, Nevalainen N, Morgan HW, Bergquist PL. Rapid cloning of thermoalkalophilic lipases from Bacillus sp. Using PCR. Biotechnol. Lett. 1999;21:1003–1006.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
