Isoform-specific regulation and localization of the coxsackie and adenovirus receptor in human airway epithelia
- PMID: 20361046
- PMCID: PMC2845650
- DOI: 10.1371/journal.pone.0009909
Isoform-specific regulation and localization of the coxsackie and adenovirus receptor in human airway epithelia
Abstract
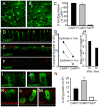
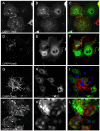
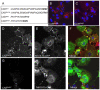
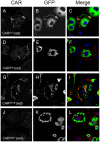
Adenovirus is an important respiratory pathogen. Adenovirus fiber from most serotypes co-opts the Coxsackie-Adenovirus Receptor (CAR) to bind and enter cells. However, CAR is a cell adhesion molecule localized on the basolateral membrane of polarized epithelia. Separation from the lumen of the airways by tight junctions renders airway epithelia resistant to inhaled adenovirus infection. Although a role for CAR in viral spread and egress has been established, the mechanism of initial respiratory infection remains controversial. CAR exists in several protein isoforms including two transmembrane isoforms that differ only at the carboxy-terminus (CAR(Ex7) and CAR(Ex8)). We found low-level expression of the CAR(Ex8) isoform in well-differentiated human airway epithelia. Surprisingly, in contrast to CAR(Ex7), CAR(Ex8) localizes to the apical membrane of epithelia where it augments adenovirus infection. Interestingly, despite sharing a similar class of PDZ-binding domain with CAR(Ex7), CAR(Ex8) differentially interacts with PICK1, PSD-95, and MAGI-1b. MAGI-1b appears to stoichiometrically regulate the degradation of CAR(Ex8) providing a potential mechanism for the apical localization of CAR(Ex8) in airway epithelial. In summary, apical localization of CAR(Ex8) may be responsible for initiation of respiratory adenoviral infections and this localization appears to be regulated by interactions with PDZ-domain containing proteins.
Conflict of interest statement
Figures




References
-
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, et al. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. - PubMed
-
- Honda T, Saitoh H, Masuko M, Katagiri-Abe T, Tominaga K, et al. The coxsackievirus-adenovirus receptor protein as a cell adhesion molecule in the developing mouse brain. Brain Res Mol Brain Res. 2000;77:19–28. - PubMed
-
- Walters R, Freimuth P, Moninger T, Ganske I, Zabner J, et al. Adenovirus Fiber Disrupts CAR-Mediated Intercellular Adhesion Allowing Virus Escape. Cell. 2002;110:789–799. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

