Fast and high-yield microreactor syntheses of ortho-substituted [(18)F]fluoroarenes from reactions of [(18)F]fluoride ion with diaryliodonium salts
- PMID: 20361793
- PMCID: PMC2891105
- DOI: 10.1021/jo100361d
Fast and high-yield microreactor syntheses of ortho-substituted [(18)F]fluoroarenes from reactions of [(18)F]fluoride ion with diaryliodonium salts
Abstract
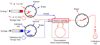
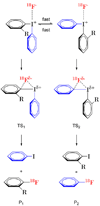
A microreactor was applied to produce ortho-substituted [(18)F]fluoroarenes from the reactions of cyclotron-produced [(18)F]fluoride ion (t(1/2) = 109.7 min) with diaryliodonium salts. The microreactor provided a very convenient means for running sequential reactions rapidly with small amounts of reagents under well-controlled conditions, thereby allowing reaction kinetics to be followed and Arrhenius activation energies (E(a)) to be measured. Prepared symmetrical iodonium chlorides (Ar(2)I(+)Cl(-)) rapidly (<4 min) gave moderate (Ar = 2-MeOC(6)H(4), 51%) to high (Ar = Ph or 2-MeC(6)H(4), 85%) decay-corrected radiochemical yields (RCYs) of a single radioactive product (Ar(18)F). Reaction velocity with respect to Ar group was 2-MeOC(6)H(4) < Ph < 2-MeC(6)H(4). Activation energies were in the range 18-28 kcal/mol. Prepared unsymmetrical salts (e.g., 2-RC(6)H(4)I(+)2'-R'C(6)H(4)X(-); X = Cl or OTs) also rapidly gave two products (2-RC(6)H(4)(18)F and 2-R'C(6)H(4)(18)F) in generally high total RCYs (79-93%). Selectivity for product [(18)F]fluoroarene was controlled by the nature of the ortho substituents. The power of ortho substituents to impart an ortho-effect was in the following order:, 2,6-di-Me > 2,4,6-tri-Me > Br > Me > Et approximately (i)Pr >> H > OMe. For (2-methyphenyl)(phenyl)iodonium chloride, the time-course of reaction product selectivity was constant and consistent with the operation of the Curtin-Hammett principle. These results will aid in the design of diaryliodonium salt precursors to (18)F-labeled tracers for molecular imaging.
Figures




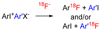

References
-
- Cai LS, Lu SY, Pike VW. Eur. J. Org. Chem. 2008;17:2853–2873.
-
- Guillaume M, Luxen A, Nebeling B, Argentini M, Clark JC, Pike VW. Appl. Radiat. Isot. 1992;42:749–762.
- Qaim SM, Clark JC, Crouzel C, Guillaume M, Helmeke HJ, Nebeling B, Pike VW, Stöcklin G. In: Radiopharmaceuticals for Positron Emission Tomography. Stöcklin G, Pike VW, editors. Dordrecht, Netherlands: Kluwer Academic Publishers; 1993. pp. 1–43.
-
- Pike VW, Aigbirhio FI. J. Chem. Soc. Chem. Commun. 1995;21:2215–2216.
-
- Shah A, Pike VW, Widdowson DA. J. Chem. Soc. Perkin Trans. 1998;1:2043–2046.
- Gail R, Hocke C, Coenen HH. J. Label. Compd. Radiopharm. 1997;40:50–52.
- Zhang MR, Kumata K, Suzuki K. Tetrahedron Lett. 2007;48:8632–8635.
- Pike VW, Aigbirhio FI. J. Label. Compd. Radiopharm. 1995;37:120–122.
-
- Martín-Santamaría S, Carroll MA, Carroll CM, Carter CD, Pike VW, Rzepa HS, Widdowson DA. Chem. Commun. 2000:649–650.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

