Development of a new generation of 4-aminoquinoline antimalarial compounds using predictive pharmacokinetic and toxicology models
- PMID: 20361799
- PMCID: PMC2866084
- DOI: 10.1021/jm100057h
Development of a new generation of 4-aminoquinoline antimalarial compounds using predictive pharmacokinetic and toxicology models
Abstract
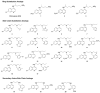
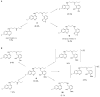
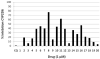
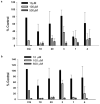
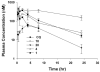
Among the known antimalarial drugs, chloroquine (CQ) and other 4-aminoquinolines have shown high potency and good bioavailability. Yet complications associated with drug resistance necessitate the discovery of effective new antimalarial agents. ADMET prediction studies were employed to evaluate a library of new molecules based on the 4-aminoquinolone-related structure of CQ. Extensive in vitro screening and in vivo pharmacokinetic studies in mice helped to identify two lead molecules, 18 and 4, with promising in vitro therapeutic efficacy, improved ADMET properties, low risk for drug-drug interactions, and desirable pharmacokinetic profiles. Both 18 and 4 are highly potent antimalarial compounds, with IC(50) values of 5.6 and 17.3 nM, respectively, against the W2 (CQ-resistant) strain of Plasmodium falciparum (for CQ, IC(50) = 382 nM). When tested in mice, these compounds were found to have biological half-lives and plasma exposure values similar to or higher than those of CQ; they are therefore desirable candidates to pursue in future clinical trials.
Figures
Similar articles
-
Design and synthesis of pyrano[2,3-c]pyrazole-4-aminoquinoline hybrids as effective antimalarial compounds.Eur J Med Chem. 2024 Dec 5;279:116828. doi: 10.1016/j.ejmech.2024.116828. Epub 2024 Sep 5. Eur J Med Chem. 2024. PMID: 39244861
-
N-Substituted aminoquinoline-pyrimidine hybrids: Synthesis, in vitro antimalarial activity evaluation and docking studies.Eur J Med Chem. 2019 Jan 15;162:277-289. doi: 10.1016/j.ejmech.2018.11.021. Epub 2018 Nov 9. Eur J Med Chem. 2019. PMID: 30448417
-
Antimalarial activity of novel 4-aminoquinolines active against drug resistant strains.Bioorg Chem. 2017 Feb;70:74-85. doi: 10.1016/j.bioorg.2016.11.010. Epub 2016 Nov 23. Bioorg Chem. 2017. PMID: 27908538
-
A medicinal chemistry perspective on 4-aminoquinoline antimalarial drugs.Curr Top Med Chem. 2006;6(5):479-507. doi: 10.2174/156802606776743147. Curr Top Med Chem. 2006. PMID: 16719804 Review.
-
Recent developments in antimalarial activities of 4-aminoquinoline derivatives.Eur J Med Chem. 2023 Aug 5;256:115458. doi: 10.1016/j.ejmech.2023.115458. Epub 2023 May 5. Eur J Med Chem. 2023. PMID: 37163950 Review.
Cited by
-
Exploring Quinoline Derivatives: Their Antimalarial Efficacy and Structural Features.Med Chem. 2025;21(2):96-121. doi: 10.2174/0115734064318361240827072124. Med Chem. 2025. PMID: 40007183 Review.
-
Evaluation of Novel α-(Acyloxy)-α-(Quinolin-4-yl) Acetamides as Antiplasmodial Agents.Iran J Pharm Res. 2017 Summer;16(3):924-928. Iran J Pharm Res. 2017. PMID: 29201083 Free PMC article.
-
Enone- and chalcone-chloroquinoline hybrid analogues: in silico guided design, synthesis, antiplasmodial activity, in vitro metabolism, and mechanistic studies.J Med Chem. 2011 May 26;54(10):3637-49. doi: 10.1021/jm200149e. Epub 2011 May 5. J Med Chem. 2011. PMID: 21500839 Free PMC article.
-
In-silico combinatorial design and pharmacophore modeling of potent antimalarial 4-anilinoquinolines utilizing QSAR and computed descriptors.Springerplus. 2015 Dec 29;4(1):819. doi: 10.1186/s40064-015-1593-3. eCollection 2015. Springerplus. 2015. PMID: 29021931 Free PMC article.
-
Reversed chloroquine molecules as a strategy to overcome resistance in malaria.Curr Top Med Chem. 2012;12(5):400-7. doi: 10.2174/156802612799362968. Curr Top Med Chem. 2012. PMID: 22242848 Free PMC article. Review.
References
-
- Kassel DB. Applications of high-throughput ADME in drug discovery. Curr Opin Chem Biol. 2004;8:339–345. - PubMed
-
- Shearer TW, Smith KS, Diaz D, Asher C, Ramirez J. The role of in vitro ADME assays in antimalarial drug discovery and development. Comb Chem High Throughput Screen. 2005;8:89–98. - PubMed
-
- WHO. World Malaria Report. World Health Organization; 2008.
-
- Madrid PB, Sherrill J, Liou AP, Weisman JL, Derisi JL, Guy RK. Synthesis of ring-substituted 4-aminoquinolines and evaluation of their antimalarial activities. Bioorg Med Chem Lett. 2005;15:1015–1018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

