Severe X-linked mitochondrial encephalomyopathy associated with a mutation in apoptosis-inducing factor
- PMID: 20362274
- PMCID: PMC2850437
- DOI: 10.1016/j.ajhg.2010.03.002
Severe X-linked mitochondrial encephalomyopathy associated with a mutation in apoptosis-inducing factor
Abstract
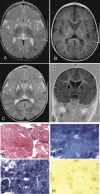
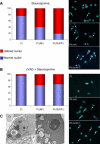
We investigated two male infant patients who were given a diagnosis of progressive mitochondrial encephalomyopathy on the basis of clinical, biochemical, and morphological features. These patients were born from monozygotic twin sisters and unrelated fathers, suggesting an X-linked trait. Fibroblasts from both showed reduction of respiratory chain (RC) cIII and cIV, but not of cI activities. We found a disease-segregating mutation in the X-linked AIFM1 gene, encoding the Apoptosis-Inducing Factor (AIF) mitochondrion-associated 1 precursor that deletes arginine 201 (R201 del). Under normal conditions, mature AIF is a FAD-dependent NADH oxidase of unknown function and is targeted to the mitochondrial intermembrane space (this form is called AIF(mit)). Upon apoptogenic stimuli, a soluble form (AIF(sol)) is released by proteolytic cleavage and migrates to the nucleus, where it induces "parthanatos," i.e., caspase-independent fragmentation of chromosomal DNA. In vitro, the AIF(R201 del) mutation decreases stability of both AIF(mit) and AIF(sol) and increases the AIF(sol) DNA binding affinity, a prerequisite for nuclear apoptosis. In AIF(R201 del) fibroblasts, staurosporine-induced parthanatos was markedly increased, whereas re-expression of AIF(wt) induced recovery of RC activities. Numerous TUNEL-positive, caspase 3-negative nuclei were visualized in patient #1's muscle, again indicating markedly increased parthanatos in the AIF(R201 del) critical tissues. We conclude that AIF(R201 del) is an unstable mutant variant associated with increased parthanatos-linked cell death. Our data suggest a role for AIF in RC integrity and mtDNA maintenance, at least in some tissues. Interestingly, riboflavin supplementation was associated with prolonged improvement of patient #1's neurological conditions, as well as correction of RC defects in mutant fibroblasts, suggesting that stabilization of the FAD binding in AIF(mit) is beneficial.
(c) 2010 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Schapira A.H. Mitochondrial disease. Lancet. 2006;368:70–82. - PubMed
-
- Smeitink J.A., Zeviani M., Turnbull D.M., Jacobs H.T. Mitochondrial medicine: A metabolic perspective on the pathology of oxidative phosphorylation disorders. Cell Metab. 2006;3:9–13. - PubMed
-
- Regev-Rudzki N., Pines O. Eclipsed distribution: A phenomenon of dual targeting of protein and its significance. Bioessays. 2007;29:772–782. - PubMed
-
- McFarland R., Turnbull D.M. Batteries not included: Diagnosis and management of mitochondrial disease. J. Intern. Med. 2009;265:210–228. - PubMed
-
- Modjtahedi N., Giordanetto F., Madeo F., Kroemer G. Apoptosis-inducing factor: Vital and lethal. Trends Cell Biol. 2006;16:264–272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

