Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial
- PMID: 20362507
- PMCID: PMC3792651
- DOI: 10.1016/S1470-2045(10)70054-1
Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial
Abstract
Background: Treatment with bisphosphonates decreases bone loss and can increase disease-free survival in patients with breast cancer. The aim of our study was to assess the effect of zoledronic acid on clearance of disseminated tumour cells (DTCs) from the bone marrow in women undergoing neoadjuvant chemotherapy for breast cancer.
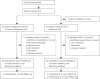
Methods: Patients were recruited for this open-label, phase 2 randomised trial between March 17, 2003, and May 19, 2006, at a single centre. Eligible patients had clinical stage II-III (> or = T2 and/or > or = N1) newly diagnosed breast cancer, Eastern Cooperative Oncology Group performance status of 0 or 1, and normal cardiac, renal, and liver function. 120 women were randomly assigned, using allocation concealment, to receive 4 mg zoledronic acid intravenously every 3 weeks (n=60), or no zoledronic acid (n=60), for 1 year concomitant with four cycles of neoadjuvant epirubicin (75 mg/m(2)) plus docetaxel (75 mg/m(2)) and two cycles of adjuvant epirubicin plus docetaxel. The primary endpoint was the number of patients with detectable DTCs at 3 months. Final analysis was done 1 year after the last patient was enrolled. Analyses were done for all patients with available data at 3 months. This study is registered with ClinicalTrials.gov, number NCT00242203.
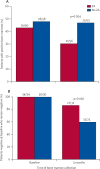
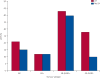
Findings: Of the 120 patients initially enrolled, one withdrew after signing consent and one patient's baseline bone marrow was not available. Both of these patients were in the control group. At 3 months, 109 bone-marrow samples were available for analysis. In the zoledronic acid group, bone marrow was not collected from one patient because of disease progression, one patient was taken off study because of severe diarrhoea, and two patients had not consented at the time of surgery. In the control group, bone marrow was not collected from two patients because of disease progression, one patient withdrew consent, and three patients were not consented at the time of surgery. At baseline, DTCs were detected in 26 of 60 patients in the zoledronic acid group and 28 of 58 patients in the control group. At 3 months, 17 of 56 patients receiving zoledronic acid versus 25 of 53 patients who did not receive zoledronic acid had detectable DTCs (p=0.054). The most common grade 3-4 toxicities were infection (five of 60 patients in the zoledronic acid group and six of 59 in the control group) and thrombosis (five of 60 in the zoledronic acid and two of 59 in the control group). There was one documented case of osteonecrosis in the zoledronic acid group.
Interpretation: Zoledronic acid administered with chemotherapy resulted in a decreased proportion of patients with DTCs detected in the bone marrow at the time of surgery. Our study supports the hypothesis that the antimetastatic effects of zoledronic acid may be through effects on DTCs.
Funding: Novartis Pharmaceuticals and Pfizer Inc.
2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
RA and KW have received honoria from Novartis. MN has received honoria from Novartis, Sanofi-Aventis, and Pfizer. ME is a consultant for Novartis and has received honoria and research funds from Novartis. KD has received honoria from Novartis. WS received funds for portions of the statistical analyses. All other authors declared no conflicts of interest.
Figures
Comment in
-
Closing in on the unknown enemy.Lancet Oncol. 2010 May;11(5):402-3. doi: 10.1016/S1470-2045(10)70091-7. Lancet Oncol. 2010. PMID: 20434707 No abstract available.
References
-
- Braun S, Vogl FD, Naume B, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353:793–802. - PubMed
-
- Braun S, Kentenich C, Janni W, et al. Lack of effect of adjuvant chemotherapy on the elimination of single dormant tumour cells in bone marrow of high-risk breast cancer patients. J Clin Oncol. 2000;18:80–86. - PubMed
-
- Pantel K, Alix-Panabieres C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol. 2009;6:339–51. - PubMed
-
- Käkönen S-M, Mundy G. Mechanisms of osteolytic bone metastases in breast carcinoma. Cancer. 2003;97:834–39. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

