Efficient derivation of functional floor plate tissue from human embryonic stem cells
- PMID: 20362538
- PMCID: PMC4336800
- DOI: 10.1016/j.stem.2010.03.001
Efficient derivation of functional floor plate tissue from human embryonic stem cells
Retraction in
-
Retraction Notice to: Efficient Derivation of Functional Floor Plate Tissue from Human Embryonic Stem Cells.Cell Stem Cell. 2023 Jun 1;30(6):905. doi: 10.1016/j.stem.2023.05.010. Cell Stem Cell. 2023. PMID: 37267920 Free PMC article. No abstract available.
Abstract
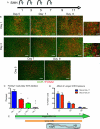
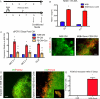
The floor plate (FP) is a critical signaling center during neural development located along the ventral midline of the embryo. Little is known about human FP development because of the lack of tissue accessibility. Here we report the efficient derivation of human embryonic stem cell (hESC)-derived FP tissue capable of secreting Netrin-1 and SHH and patterning primary and hESC derived tissues. FP induction in hESCs is dependent on early SHH exposure and occurs at the expense of anterior neurectoderm (AN). Global gene expression and functional studies identify SHH-mediated inhibition of Dkk-1 as key factor in FP versus AN specification. hESC-derived FP tissue is shown to be of anterior SIX6+ character but is responsive to caudalizing factors suppressing SIX6 expression and inducing a shift in usage of region-specific SHH enhancers. These data define the early signals that drive human FP versus AN specification and determine regional identity in hESC-derived FP.
Copyright c) 2010 Elsevier Inc. All rights reserved.
Figures




References
-
- Barberi T, Klivenyi P, Calingasan NY, Lee H, Kawamata H, Loonam K, Perrier AL, Bruses J, Rubio ME, Topf N, Tabar V, Harrison NL, Beal MF, Moore MA, Studer L. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol. 2003;10:1200–1207. - PubMed
-
- Briscoe J, Ericson J. The specification of neuronal identity by graded Sonic Hedgehog signaling. Semin Cell Dev Biol. 1999;3:353–62. - PubMed
-
- Charrier JB, Lapointe F, Le Douarin NM, Teillet MA. Dual origin of the floor plate in the avian embryo. Development. 2002;129:4785–4796. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

