Safety and efficacy of lithium in combination with riluzole for treatment of amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial
- PMID: 20363190
- PMCID: PMC3071495
- DOI: 10.1016/S1474-4422(10)70068-5
Safety and efficacy of lithium in combination with riluzole for treatment of amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial
Abstract
Background: In a pilot study, lithium treatment slowed progression of amyotrophic lateral sclerosis (ALS). We aimed to confirm or disprove these findings by assessing the safety and efficacy of lithium in combination with riluzole in patients with ALS.
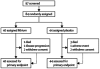
Methods: We did a double-blind, placebo-controlled trial with a time-to-event design. Between January and June, 2009, patients with ALS who were taking a stable dose of riluzole for at least 30 days were randomly assigned (1:1) by a centralised computer to receive either lithium or placebo. Patients, caregivers, investigators, and all site study staff with the exception of site pharmacists were masked to treatment assignment. The primary endpoint was the time to an event, defined as a decrease of at least six points on the revised ALS functional rating scale score or death. Interim analyses were planned for when 84 patients had been allocated treatment, 6 months later or after 55 events, and after 100 events. Analysis was by intention to treat. The stopping boundary for futility at the first interim analysis was a p value of at least 0.68. We used a log-rank test to compare the distributions of the time to an event between the lithium and placebo groups. This trial is registered with ClinicalTrials.gov, NCT00818389.
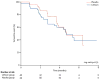
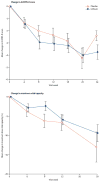
Findings: At the first interim analysis, 22 of 40 patients in the lithium group had an event compared with 20 of 44 patients in the placebo group (log rank p=0.51). The hazard ratio of reaching the primary endpoint was 1.13 (95% CI 0.61-2.07). The study was stopped at the first interim analysis because criterion for futility was met (p=0.78). The difference in mean decline in the ALS functional rating scale score between the lithium group and the placebo group was 0.15 (95% CI -0.43 to 0.73, p=0.61). There were no major safety concerns. Falls (p=0.04) and back pain (p=0.05) were more common in the lithium group than in the placebo group.
Interpretation: We found no evidence that lithium in combination with riluzole slows progression of ALS more than riluzole alone. The time-to-event endpoint and use of prespecified interim analyses enabled a clear result to be obtained rapidly. This design should be considered for future trials testing the therapeutic efficacy of drugs that are easily accessible to people with ALS.
Funding: National Institute of Neurological Disorders and Stroke, ALS Association, and ALS Society of Canada.
Copyright 2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures

Comment in
-
Lithium time-to-event trial in amyotrophic lateral sclerosis stops early for futility.Lancet Neurol. 2010 May;9(5):449-51. doi: 10.1016/S1474-4422(10)70085-5. Epub 2010 Apr 1. Lancet Neurol. 2010. PMID: 20363191 No abstract available.
-
Would riluzole be efficacious in the new ALS trial design?Lancet Neurol. 2010 Oct;9(10):949-50; author reply 950-1. doi: 10.1016/S1474-4422(10)70230-1. Lancet Neurol. 2010. PMID: 20864047 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

