Features of a spatially constrained cystine loop in the p10 FAST protein ectodomain define a new class of viral fusion peptides
- PMID: 20363742
- PMCID: PMC2878076
- DOI: 10.1074/jbc.M110.118232
Features of a spatially constrained cystine loop in the p10 FAST protein ectodomain define a new class of viral fusion peptides
Abstract
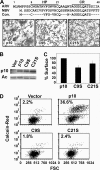
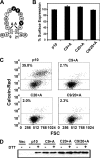
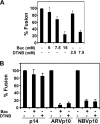
The reovirus fusion-associated small transmembrane (FAST) proteins are the smallest known viral membrane fusion proteins. With ectodomains of only approximately 20-40 residues, it is unclear how such diminutive fusion proteins can mediate cell-cell fusion and syncytium formation. Contained within the 40-residue ectodomain of the p10 FAST protein resides an 11-residue sequence of moderately apolar residues, termed the hydrophobic patch (HP). Previous studies indicate the p10 HP shares operational features with the fusion peptide motifs found within the enveloped virus membrane fusion proteins. Using biotinylation assays, we now report that two highly conserved cysteine residues flanking the p10 HP form an essential intramolecular disulfide bond to create a cystine loop. Mutagenic analyses revealed that both formation of the cystine loop and p10 membrane fusion activity are highly sensitive to changes in the size and spatial arrangement of amino acids within the loop. The p10 cystine loop may therefore function as a cystine noose, where fusion peptide activity is dependent on structural constraints within the noose that force solvent exposure of key hydrophobic residues. Moreover, inhibitors of cell surface thioreductase activity indicate that disruption of the disulfide bridge is important for p10-mediated membrane fusion. This is the first example of a viral fusion peptide composed of a small, spatially constrained cystine loop whose function is dependent on altered loop formation, and it suggests the p10 cystine loop represents a new class of viral fusion peptides.
Figures


Similar articles
-
The p10 FAST protein fusion peptide functions as a cystine noose to induce cholesterol-dependent liposome fusion without liposome tubulation.Biochim Biophys Acta. 2015 Feb;1848(2):408-16. doi: 10.1016/j.bbamem.2014.10.020. Epub 2014 Oct 23. Biochim Biophys Acta. 2015. PMID: 25450808
-
A compact, multifunctional fusion module directs cholesterol-dependent homomultimerization and syncytiogenic efficiency of reovirus p10 FAST proteins.PLoS Pathog. 2014 Mar 20;10(3):e1004023. doi: 10.1371/journal.ppat.1004023. eCollection 2014 Mar. PLoS Pathog. 2014. PMID: 24651689 Free PMC article.
-
Myristoylation, a protruding loop, and structural plasticity are essential features of a nonenveloped virus fusion peptide motif.J Biol Chem. 2004 Dec 3;279(49):51386-94. doi: 10.1074/jbc.M406990200. Epub 2004 Sep 24. J Biol Chem. 2004. PMID: 15448165
-
Reovirus FAST proteins: virus-encoded cellular fusogens.Trends Microbiol. 2014 Dec;22(12):715-24. doi: 10.1016/j.tim.2014.08.005. Epub 2014 Sep 19. Trends Microbiol. 2014. PMID: 25245455 Review.
-
The role of the transmembrane and of the intraviral domain of glycoproteins in membrane fusion of enveloped viruses.Biosci Rep. 2000 Dec;20(6):571-95. doi: 10.1023/a:1010415122234. Biosci Rep. 2000. PMID: 11426695 Review.
Cited by
-
Rotavirus Species B Encodes a Functional Fusion-Associated Small Transmembrane Protein.J Virol. 2019 Sep 30;93(20):e00813-19. doi: 10.1128/JVI.00813-19. Print 2019 Oct 15. J Virol. 2019. PMID: 31375572 Free PMC article.
-
Cell-cell membrane fusion induced by p15 fusion-associated small transmembrane (FAST) protein requires a novel fusion peptide motif containing a myristoylated polyproline type II helix.J Biol Chem. 2012 Jan 27;287(5):3403-14. doi: 10.1074/jbc.M111.305268. Epub 2011 Dec 14. J Biol Chem. 2012. PMID: 22170056 Free PMC article.
-
Polycistronic Genome Segment Evolution and Gain and Loss of FAST Protein Function during Fusogenic Orthoreovirus Speciation.Viruses. 2020 Jun 29;12(7):702. doi: 10.3390/v12070702. Viruses. 2020. PMID: 32610593 Free PMC article.
-
Cell-cell fusion induced by reovirus FAST proteins enhances replication and pathogenicity of non-enveloped dsRNA viruses.PLoS Pathog. 2019 Apr 25;15(4):e1007675. doi: 10.1371/journal.ppat.1007675. eCollection 2019 Apr. PLoS Pathog. 2019. PMID: 31022290 Free PMC article.
-
Efficient reovirus- and measles virus-mediated pore expansion during syncytium formation is dependent on annexin A1 and intracellular calcium.J Virol. 2014 Jun;88(11):6137-47. doi: 10.1128/JVI.00121-14. Epub 2014 Mar 19. J Virol. 2014. PMID: 24648446 Free PMC article.
References
-
- Martens S., McMahon H. T. (2008) Nat. Rev. Mol. Cell Biol. 9, 543–556 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

