Activation of glucose-6-phosphate dehydrogenase promotes acute hypoxic pulmonary artery contraction
- PMID: 20363753
- PMCID: PMC2885235
- DOI: 10.1074/jbc.M109.092916
Activation of glucose-6-phosphate dehydrogenase promotes acute hypoxic pulmonary artery contraction
Abstract
Hypoxic pulmonary vasoconstriction (HPV) is a physiological response to a decrease in airway O(2) tension, but the underlying mechanism is incompletely understood. We studied the contribution of glucose-6-phosphate dehydrogenase (Glc-6-PD), an important regulator of NADPH redox and production of reactive oxygen species, to the development of HPV. We found that hypoxia (95% N(2), 5% CO(2)) increased contraction of bovine pulmonary artery (PA) precontracted with KCl or serotonin. Depletion of extracellular glucose reduced NADPH, NADH, and HPV, substantiating the idea that glucose metabolism and Glc-6-PD play roles in the response of PA to hypoxia. Our data also show that inhibition of glycolysis and mitochondrial respiration (indicated by an increase in NAD(+) and decrease in the ATP-to-ADP ratio) by hypoxia, or by inhibitors of pyruvate dehydrogenase or electron transport chain complexes I or III, increased generation of reactive oxygen species, which in turn activated Glc-6-PD. Inhibition of Glc-6-PD decreased Ca(2+) sensitivity to the myofilaments and diminished Ca(2+)-independent and -dependent myosin light chain phosphorylation otherwise increased by hypoxia. Silencing Glc-6-PD expression in PA using a targeted small interfering RNA abolished HPV and decreased extracellular Ca(2+)-dependent PA contraction increased by hypoxia. Similarly, Glc-6-PD expression and activity were significantly reduced in lungs from Glc-6-PD(mut(-/-)) mice, and there was a corresponding reduction in HPV. Finally, regression analysis relating Glc-6-PD activity and the NADPH-to-NADP(+) ratio to the HPV response clearly indicated a positive linear relationship between Glc-6-PD activity and HPV. Based on these findings, we propose that Glc-6-PD and NADPH redox are crucially involved in the mechanism of HPV and, in turn, may play a key role in increasing pulmonary arterial pressure, which is involved in the development of pulmonary hypertension.
Figures

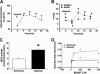

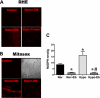






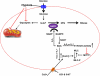
 ) anion and/or H2O2 activates RhoA-ROCK and inhibits myosin light chain phosphate (MYPT1), enhances intracellular [Ca2+]i via influx and release, and increases myosin light chain phosphorylation (MLC-P). Increase in MLC phosphorylation causes contraction of PASMC and promotes hypoxic pulmonary vasoconstriction.
) anion and/or H2O2 activates RhoA-ROCK and inhibits myosin light chain phosphate (MYPT1), enhances intracellular [Ca2+]i via influx and release, and increases myosin light chain phosphorylation (MLC-P). Increase in MLC phosphorylation causes contraction of PASMC and promotes hypoxic pulmonary vasoconstriction.Similar articles
-
Glc-6-PD and PKG contribute to hypoxia-induced decrease in smooth muscle cell contractile phenotype proteins in pulmonary artery.Am J Physiol Lung Cell Mol Physiol. 2012 Jul 1;303(1):L64-74. doi: 10.1152/ajplung.00002.2012. Epub 2012 May 11. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22582112 Free PMC article.
-
Role of pentose phosphate pathway-derived NADPH in hypoxic pulmonary vasoconstriction.Pulm Pharmacol Ther. 2006;19(4):303-9. doi: 10.1016/j.pupt.2005.08.002. Epub 2005 Oct 3. Pulm Pharmacol Ther. 2006. PMID: 16203165
-
Divergent roles of glycolysis and the mitochondrial electron transport chain in hypoxic pulmonary vasoconstriction of the rat: identity of the hypoxic sensor.J Physiol. 2001 Oct 1;536(Pt 1):211-24. doi: 10.1111/j.1469-7793.2001.00211.x. J Physiol. 2001. PMID: 11579170 Free PMC article.
-
Oxidant-redox regulation of pulmonary vascular responses to hypoxia and nitric oxide-cGMP signaling.Cardiol Rev. 2010 Mar-Apr;18(2):89-93. doi: 10.1097/CRD.0b013e3181c9f088. Cardiol Rev. 2010. PMID: 20160535 Free PMC article. Review.
-
Molecular identification of O2 sensors and O2-sensitive potassium channels in the pulmonary circulation.Adv Exp Med Biol. 2000;475:219-40. doi: 10.1007/0-306-46825-5_21. Adv Exp Med Biol. 2000. PMID: 10849663 Review.
Cited by
-
Redox Mechanisms Influencing cGMP Signaling in Pulmonary Vascular Physiology and Pathophysiology.Adv Exp Med Biol. 2017;967:227-240. doi: 10.1007/978-3-319-63245-2_13. Adv Exp Med Biol. 2017. PMID: 29047089 Free PMC article.
-
Dehydroepiandrosterone promotes pulmonary artery relaxation by NADPH oxidation-elicited subunit dimerization of protein kinase G 1α.Am J Physiol Lung Cell Mol Physiol. 2014 Feb 15;306(4):L383-91. doi: 10.1152/ajplung.00301.2013. Epub 2013 Dec 27. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24375799 Free PMC article.
-
Redox regulation of guanylate cyclase and protein kinase G in vascular responses to hypoxia.Respir Physiol Neurobiol. 2010 Dec 31;174(3):259-64. doi: 10.1016/j.resp.2010.08.024. Epub 2010 Sep 8. Respir Physiol Neurobiol. 2010. PMID: 20831906 Free PMC article. Review.
-
Sulfhydryl-dependent dimerization of soluble guanylyl cyclase modulates the relaxation of porcine pulmonary arteries to nitric oxide.Pflugers Arch. 2013 Feb;465(2):333-41. doi: 10.1007/s00424-012-1176-x. Epub 2012 Nov 10. Pflugers Arch. 2013. PMID: 23143201
-
Redox Regulation of Ion Channels and Receptors in Pulmonary Hypertension.Antioxid Redox Signal. 2019 Oct 20;31(12):898-915. doi: 10.1089/ars.2018.7699. Epub 2019 Jan 25. Antioxid Redox Signal. 2019. PMID: 30569735 Free PMC article. Review.
References
-
- Sommer N., Dietrich A., Schermuly R. T., Ghofrani H. A., Gudermann T., Schulz R., Seeger W., Grimminger F., Weissmann N. (2008) Eur. Respir. J. 32, 1639–1651 - PubMed
-
- Stenmark K. R., Fagan K. A., Frid M. G. (2006) Circ. Res. 99, 675–691 - PubMed
-
- McMurtry I. F., Rounds S., Stanbrook H. S. (1982) Adv. Shock Res. 8, 21–33 - PubMed
-
- Stanbrook H. S., McMurtry I. F. (1983) J. Appl. Physiol. 55, 1467–1473 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

