Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling
- PMID: 20363774
- PMCID: PMC3324297
- DOI: 10.1182/blood-2010-02-270876
Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling
Abstract
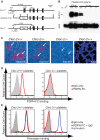
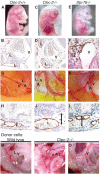
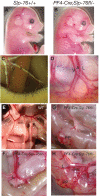
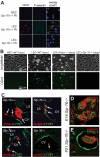
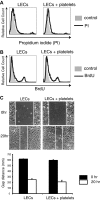
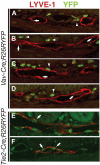
Although platelets appear by embryonic day 10.5 in the developing mouse, an embryonic role for these cells has not been identified. The SYK-SLP-76 signaling pathway is required in blood cells to regulate embryonic blood-lymphatic vascular separation, but the cell type and molecular mechanism underlying this regulatory pathway are not known. In the present study we demonstrate that platelets regulate lymphatic vascular development by directly interacting with lymphatic endothelial cells through C-type lectin-like receptor 2 (CLEC-2) receptors. PODOPLANIN (PDPN), a transmembrane protein expressed on the surface of lymphatic endothelial cells, is required in nonhematopoietic cells for blood-lymphatic separation. Genetic loss of the PDPN receptor CLEC-2 ablates PDPN binding by platelets and confers embryonic lymphatic vascular defects like those seen in animals lacking PDPN or SLP-76. Platelet factor 4-Cre-mediated deletion of Slp-76 is sufficient to confer lymphatic vascular defects, identifying platelets as the cell type in which SLP-76 signaling is required to regulate lymphatic vascular development. Consistent with these genetic findings, we observe SLP-76-dependent platelet aggregate formation on the surface of lymphatic endothelial cells in vivo and ex vivo. These studies identify a nonhemostatic pathway in which platelet CLEC-2 receptors bind lymphatic endothelial PDPN and activate SLP-76 signaling to regulate embryonic vascular development.
Figures

Comment in
-
Inside bloody lymphatics.Blood. 2010 Jul 29;116(4):512-3. doi: 10.1182/blood-2010-04-278549. Blood. 2010. PMID: 20671134 No abstract available.
References
-
- Shalaby F, Rossant J, Yamaguchi TP, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376(6535):62–66. - PubMed
-
- Stainier DY, Weinstein BM, Detrich HW, III, Zon LI, Fishman MC. Cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development. 1995;121(10):3141–3150. - PubMed
-
- Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9(6):702–712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

