Epithelial repair mechanisms in the lung
- PMID: 20363851
- PMCID: PMC2886606
- DOI: 10.1152/ajplung.00361.2009
Epithelial repair mechanisms in the lung
Abstract
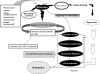
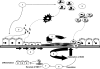
The recovery of an intact epithelium following lung injury is critical for restoration of lung homeostasis. The initial processes following injury include an acute inflammatory response, recruitment of immune cells, and epithelial cell spreading and migration upon an autologously secreted provisional matrix. Injury causes the release of factors that contribute to repair mechanisms including members of the epidermal growth factor and fibroblast growth factor families (TGF-alpha, KGF, HGF), chemokines (MCP-1), interleukins (IL-1beta, IL-2, IL-4, IL-13), and prostaglandins (PGE(2)), for example. These factors coordinate processes involving integrins, matrix materials (fibronectin, collagen, laminin), matrix metalloproteinases (MMP-1, MMP-7, MMP-9), focal adhesions, and cytoskeletal structures to promote cell spreading and migration. Several key signaling pathways are important in regulating these processes, including sonic hedgehog, Rho GTPases, MAP kinase pathways, STAT3, and Wnt. Changes in mechanical forces may also affect these pathways. Both localized and distal progenitor stem cells are recruited into the injured area, and proliferation and phenotypic differentiation of these cells leads to recovery of epithelial function. Persistent injury may contribute to the pathology of diseases such as asthma, chronic obstructive pulmonary disease, and pulmonary fibrosis. For example, dysregulated repair processes involving TGF-beta and epithelial-mesenchymal transition may lead to fibrosis. This review focuses on the processes of epithelial restitution, the localization and role of epithelial progenitor stem cells, the initiating factors involved in repair, and the signaling pathways involved in these processes.
Figures
References
-
- Adamson IY, Bowden DH. Derivation of type 1 epithelium from type 2 cells in the developing rat lung. Lab Invest 3 2: 736–745, 1975 - PubMed
-
- Ahdieh M, Vandenbos T, Youakim A. Lung epithelial barrier function and wound healing are decreased by IL-4 and IL-13 and enhanced by IFN-gamma. Am J Physiol Cell Physiol 281: C2029–C2038, 2001 - PubMed
-
- Atabai K, Ishigaki M, Geiser T, Ueki I, Matthay MA, Ware LB. Keratinocyte growth factor can enhance alveolar epithelial repair by nonmitogenic mechanisms. Am J Physiol Lung Cell Mol Physiol 283: L163–L169, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

