Human recombinant vascular endothelial growth factor reduces necrosis and enhances hepatocyte regeneration in a mouse model of acetaminophen toxicity
- PMID: 20363854
- PMCID: PMC2912052
- DOI: 10.1124/jpet.109.163840
Human recombinant vascular endothelial growth factor reduces necrosis and enhances hepatocyte regeneration in a mouse model of acetaminophen toxicity
Abstract
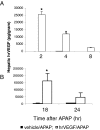
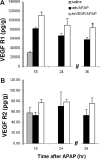
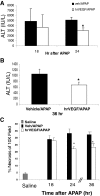
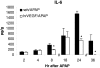
We reported previously that vascular endothelial growth factor (VEGF) was increased in acetaminophen (APAP) toxicity in mice and treatment with a VEGF receptor inhibitor reduced hepatocyte regeneration. The effect of human recombinant VEGF (hrVEGF) on APAP toxicity in the mouse was examined. In early toxicity studies, B6C3F1 mice received hrVEGF (50 microg s.c.) or vehicle 30 min before receiving APAP (200 mg/kg i.p.) and were sacrificed at 2, 4, and 8 h. Toxicity was comparable at 2 and 4 h, but reduced in the APAP/hrVEGF mice at 8 h (p < 0.05) compared with the APAP/vehicle mice. Hepatic glutathione (GSH) and APAP protein adduct levels were comparable between the two groups of mice, with the exception that GSH was higher at 8 h in the hrVEGF-treated mice. Subsequently, mice received two doses (before and 10 h) or three doses (before and 10 and 24 h) of hrVEGF; alanine aminotransferase values and necrosis were reduced at 24 and 36 h, respectively, in the APAP/hrVEGF mice (p < 0.05) compared with the APAP/vehicle mice. Proliferating cell nuclear antigen expression was enhanced, and interleukin-6 expression was reduced in the mice that received hrVEGF (p < 0.05) compared with the APAP/vehicle mice. In addition, treatment with hrVEGF lowered plasma hyaluronic acid levels and neutrophil counts at 36 h. Cumulatively, the data show that treatment with hrVEGF reduced toxicity and increased hepatocyte regeneration in APAP toxicity in the mouse. Attenuation of sinusoidal cell endothelial dysfunction and changes in neutrophil dynamics may be operant mechanisms in the hepatoprotection mediated by hrVEGF in APAP toxicity.
Figures





References
-
- Bajt ML, Knight TR, Farhood A, Jaeschke H. (2003) Scavenging peroxynitrite with glutathione promotes regeneration and enhances survival during acetaminophen-induced liver injury in mice. J Pharmacol Exp Ther 307:67–73 - PubMed
-
- Banai S, Jaklitsch MT, Shou M, Lazarous DF, Scheinowitz M, Biro S, Epstein SE, Unger EF. (1994) Angiogenic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth factor in dogs. Circulation 89:2183–2189 - PubMed
-
- Bourdi M, Masubuchi Y, Reilly TP, Amouzadeh HR, Martin JL, George JW, Shah AG, Pohl LR. (2002) Protection against acetaminophen-induced liver injury and lethality by interleukin 10: role of inducible nitric oxide synthase. Hepatology 35:289–298 - PubMed
-
- Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. (1996) Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 274:1379–1383 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

