Properties of WT and mutant hERG K(+) channels expressed in neonatal mouse cardiomyocytes
- PMID: 20363883
- PMCID: PMC2886621
- DOI: 10.1152/ajpheart.01236.2009
Properties of WT and mutant hERG K(+) channels expressed in neonatal mouse cardiomyocytes
Abstract
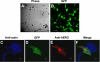
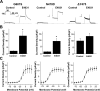
Mutations in human ether-a-go-go-related gene 1 (hERG) are linked to long QT syndrome type 2 (LQT2). hERG encodes the pore-forming alpha-subunits that coassemble to form rapidly activating delayed rectifier K(+) current in the heart. LQT2-linked missense mutations have been extensively studied in noncardiac heterologous expression systems, where biogenic (protein trafficking) and biophysical (gating and permeation) abnormalities have been postulated to underlie the loss-of-function phenotype associated with LQT2 channels. Little is known about the properties of LQT2-linked hERG channel proteins in native cardiomyocyte systems. In this study, we expressed wild-type (WT) hERG and three LQT2-linked mutations in neonatal mouse cardiomyocytes and studied their electrophysiological and biochemical properties. Compared with WT hERG channels, the LQT2 missense mutations G601S and N470D hERG exhibited altered protein trafficking and underwent pharmacological correction, and N470D hERG channels gated at more negative voltages. The DeltaY475 hERG deletion mutation trafficked similar to WT hERG channels, gated at more negative voltages, and had rapid deactivation kinetics, and these properties were confirmed in both neonatal mouse cardiomyocyte and human embryonic kidney (HEK)-293 cell expression systems. Differences between the cardiomyocytes and HEK-293 cell expression systems were that hERG current densities were reduced 10-fold and deactivation kinetics were accelerated 1.5- to 2-fold in neonatal mouse cardiomyocytes. An important finding of this work is that pharmacological correction of trafficking-deficient LQT2 mutations, as a potential innovative approach to therapy, is possible in native cardiac tissue.
Figures



References
-
- Akhavan A, Atanasiu R, Noguchi T, Han W, Holder N, Shrier A. Identification of the cyclic-nucleotide-binding domain as a conserved determinant of ion-channel cell-surface localization. J Cell Sci 118: 2803–2812, 2005 - PubMed
-
- Anderson CL, Delisle BP, Anson BD, Kilby JA, Will ML, Tester DJ, Gong Q, Zhou Z, Ackerman MJ, January CT. Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation 113: 365–373, 2006 - PubMed
-
- Anson BD, Ackerman MJ, Tester DJ, Will ML, Delisle BP, Anderson CL, January CT. Molecular and functional characterization of common polymorphisms in HERG (KCNH2) potassium channels. Am J Physiol Heart Circ Physiol 286: H2434–H2441, 2004 - PubMed
-
- Babij P, Askew GR, Nieuwenhuijsen B, Su CM, Bridal TR, Jow B, Argentieri TM, Kulik J, DeGennaro LJ, Spinelli W, Colatsky TJ. Inhibition of cardiac delayed rectifier K+ current by overexpression of the long-QT syndrome HERG G628S mutation in transgenic mice. Circ Res 83: 668–678, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

