Myocardial adenosine A(1)-receptor-mediated adenoprotection involves phospholipase C, PKC-epsilon, and p38 MAPK, but not HSP27
- PMID: 20363896
- PMCID: PMC2886631
- DOI: 10.1152/ajpheart.01028.2009
Myocardial adenosine A(1)-receptor-mediated adenoprotection involves phospholipase C, PKC-epsilon, and p38 MAPK, but not HSP27
Abstract
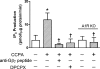
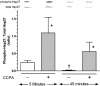
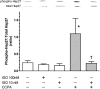
Adenosine via an adenosine A(1) receptor (A(1)R) is a negative feedback inhibitor of adrenergic stimulation in the heart, protecting it from toxic effects of overstimulation. Stimulation of the A(1)R results in the activation of G(i) protein, release of free Gbetagamma-subunits, and activation/translocation of PKC-epsilon to the receptor for activated C kinase 2 protein at the Z-line of the cardiomyocyte sarcomere. Using an anti-Gbetagamma peptide, we investigated the role of these subunits in the A(1)R stimulation of phospholipase C (PLC), with the premise that the resulting diacylglycerol provides for the activation of PKC-epsilon. Inositol 1,4,5-triphosphate release was an index of PLC activity. Chlorocyclopentyl adenosine (CCPA), an A(1)R agonist, increased inositol 1,4,5-triphosphate production by 273% in mouse heart homogenates, an effect absent in A(1)R knockout hearts and inhibited by anti-Gbetagamma peptide. In a second study, p38 MAPK and heat shock protein 27 (HSP27), found by others to be associated with the loss of myocardial contractile function, were postulated to play a role in the actions of A(1)R. Isoproterenol, a beta-adrenergic receptor agonist, increased the Ca(2+) transient and sarcomere shortening magnitudes by 36 and 49%, respectively. In the rat cardiomyocyte, CCPA significantly reduced these increases, an action blocked by the p38 MAPK inhibitor SB-203580. While CCPA significantly increased the phosphorylation of HSP27, this action was inhibited by isoproterenol. These data indicate that the activation of PKC-epsilon by A(1)R results from the activation of PLC via free Gbetagamma-subunits released upon A(1)R-induced dissociation of G(i)alphabetagamma. Attenuation of beta-adrenergic-induced contractile function by A(1)R may involve the activation of p38 MAPK, but not HSP27.
Figures


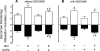

Similar articles
-
Crosstalk between adenosine A1 and β1-adrenergic receptors regulates translocation of PKCε in isolated rat cardiomyocytes.J Cell Physiol. 2012 Sep;227(9):3201-7. doi: 10.1002/jcp.24008. J Cell Physiol. 2012. PMID: 22105697 Free PMC article.
-
Adenoprotection of the heart involves phospholipase C-induced activation and translocation of PKC-epsilon to RACK2 in adult rat and mouse.Am J Physiol Heart Circ Physiol. 2009 Aug;297(2):H718-25. doi: 10.1152/ajpheart.00247.2009. Epub 2009 Jun 12. Am J Physiol Heart Circ Physiol. 2009. PMID: 19525381 Free PMC article.
-
A1 adenosine receptor attenuates intracerebral hemorrhage-induced secondary brain injury in rats by activating the P38-MAPKAP2-Hsp27 pathway.Mol Brain. 2016 Jun 14;9(1):66. doi: 10.1186/s13041-016-0247-x. Mol Brain. 2016. PMID: 27301321 Free PMC article.
-
Adenosine A(1) receptor induced delayed preconditioning in rabbits: induction of p38 mitogen-activated protein kinase activation and Hsp27 phosphorylation via a tyrosine kinase- and protein kinase C-dependent mechanism.Circ Res. 2000 May 12;86(9):989-97. doi: 10.1161/01.res.86.9.989. Circ Res. 2000. PMID: 10807872
-
Adenosine A2A and beta-adrenergic calcium transient and contractile responses in rat ventricular myocytes.Am J Physiol Heart Circ Physiol. 2008 Dec;295(6):H2364-72. doi: 10.1152/ajpheart.00927.2008. Epub 2008 Oct 10. Am J Physiol Heart Circ Physiol. 2008. PMID: 18849328 Free PMC article.
Cited by
-
Crosstalk between adenosine A1 and β1-adrenergic receptors regulates translocation of PKCε in isolated rat cardiomyocytes.J Cell Physiol. 2012 Sep;227(9):3201-7. doi: 10.1002/jcp.24008. J Cell Physiol. 2012. PMID: 22105697 Free PMC article.
-
Neuronal Adenosine A1 Receptor is Critical for Olfactory Function but Unable to Attenuate Olfactory Dysfunction in Neuroinflammation.Front Cell Neurosci. 2022 Jun 30;16:912030. doi: 10.3389/fncel.2022.912030. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35846561 Free PMC article.
-
ARF1-regulated coatomer directs the steady-state localization of protein kinase C epsilon at the Golgi apparatus.Biochim Biophys Acta. 2013 Mar;1833(3):487-93. doi: 10.1016/j.bbamcr.2012.11.011. Epub 2012 Nov 27. Biochim Biophys Acta. 2013. PMID: 23195223 Free PMC article.
-
Regulation of Circadian Genes by the MAPK Pathway: Implications for Rapid Antidepressant Action.Neurosci Bull. 2020 Jan;36(1):66-76. doi: 10.1007/s12264-019-00358-9. Epub 2019 Mar 11. Neurosci Bull. 2020. PMID: 30859414 Free PMC article. Review.
-
Reduced adenosine release from the aged mammalian heart.J Cell Physiol. 2012 Nov;227(11):3709-14. doi: 10.1002/jcp.24079. J Cell Physiol. 2012. PMID: 22378276 Free PMC article.
References
-
- Chang M, Zhang L, Tam JP, Sanders-Bush E. Dissecting G protein-coupled receptor signaling pathways with membrane-permeable blocking peptides. J Biol Chem 275: 7021–7029, 2000 - PubMed
-
- Chen Y, Rajashree R, Liu Q, Hofmann P. Acute p38 MAPK activation decreases force development in ventricular myocytes. Am J Physiol Heart Circ Physiol 285: H2578–H2586, 2003 - PubMed
-
- Communal C, Colucci WS, Singh K. p38 Mitogen-activated protein kinase pathway protects adult rat ventricular myocytes against β-adrenergic receptor stimulated apoptosis. J Biol Chem 275: 19395–19400, 2000 - PubMed
-
- Dobson JG., Jr Reduction by adenosine of the isoproterenol-induced increase in cyclic adenosine 3′,5′-monophosphate formation and glycogen phosphorylase activity in rat heart muscle. Circ Res 43: 785–792, 1978 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

