Impaired gastric gland differentiation in Peutz-Jeghers syndrome
- PMID: 20363912
- PMCID: PMC2861111
- DOI: 10.2353/ajpath.2010.090519
Impaired gastric gland differentiation in Peutz-Jeghers syndrome
Abstract
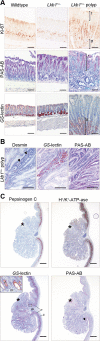
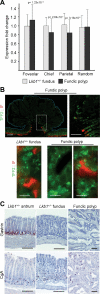
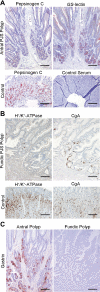
Gastrointestinal hamartomatous polyps in the Peutz-Jeghers cancer predisposition syndrome and its mouse model (Lkb1(+/-)) are presumed to contain all cell types native to the site of their occurrence. This study aimed to explore the pathogenesis of Peutz-Jeghers syndrome polyposis by characterizing cell types and differentiation of the epithelium of gastric polyps and predisposed mucosa. Both antral and fundic polyps were characterized by a deficit of pepsinogen C-expressing differentiated gland cells (antral gland, mucopeptic, and chief cells); in large fundic polyps, parietal cells were also absent. Gland cell loss was associated with an increase in precursor neck cells, an expansion of the proliferative zone, and an increase in smooth muscle alpha-actin expressing myofibroblasts in the polyp stroma. Lack of pepsinogen C-positive gland cells identified incipient polyps, and even the unaffected mucosa of young predisposed mice displayed an increase in pepsinogen C negative glands (25%; P = 0045). In addition, in small intestinal polyps, gland cell differentiation was defective, with the absence of Paneth cells. There were no signs of metaplastic differentiation in any of the tissues studied, and both the gastric and small intestinal defects were seen in Lkb1(+/-) mice, as well as polyps from patients with Peutz-Jeghers syndrome. These results identify impaired epithelial differentiation as the earliest pathological sign likely to contribute to tumorigenesis in individuals with inherited Lkb1 mutations.
Figures



Similar articles
-
Solitary Peutz-Jeghers Type Polyp of Jejunum with Gastric Fundic and Antral Gland Lining Mucosa: A Case Report and Review of Literature.Int J Surg Pathol. 2022 Aug;30(5):539-542. doi: 10.1177/10668969211067760. Epub 2021 Dec 27. Int J Surg Pathol. 2022. PMID: 34955063 Review.
-
Elevation of WNT5A expression in polyp formation in Lkb1+/- mice and Peutz-Jeghers syndrome.J Pathol. 2011 Apr;223(5):584-92. doi: 10.1002/path.2835. Epub 2011 Feb 21. J Pathol. 2011. PMID: 21341271
-
Immunolocalization of beta catenin in intestinal polyps of Peutz-Jeghers and juvenile polyposis syndromes.J Clin Pathol. 1999 May;52(5):345-9. doi: 10.1136/jcp.52.5.345. J Clin Pathol. 1999. PMID: 10560353 Free PMC article.
-
Morphologic characterization of hamartomatous gastrointestinal polyps in Cowden syndrome, Peutz-Jeghers syndrome, and juvenile polyposis syndrome.Hum Pathol. 2016 Mar;49:39-48. doi: 10.1016/j.humpath.2015.10.002. Epub 2015 Oct 31. Hum Pathol. 2016. PMID: 26826408
-
Hamartomatous polyps - a clinical and molecular genetic study.Dan Med J. 2016 Aug;63(8):B5280. Dan Med J. 2016. PMID: 27477802 Review.
Cited by
-
LKB1 as the ghostwriter of crypt history.Fam Cancer. 2011 Sep;10(3):437-46. doi: 10.1007/s10689-011-9469-3. Fam Cancer. 2011. PMID: 21805166 Free PMC article. Review.
-
Is surveillance colonoscopy necessary for patients with sporadic gastric hyperplastic polyps?PLoS One. 2015 Apr 13;10(4):e0122996. doi: 10.1371/journal.pone.0122996. eCollection 2015. PLoS One. 2015. PMID: 25874940 Free PMC article.
-
The Peutz-Jeghers kinase LKB1 suppresses polyp growth from intestinal cells of a proglucagon-expressing lineage in mice.Dis Model Mech. 2014 Nov;7(11):1275-86. doi: 10.1242/dmm.014720. Epub 2014 Sep 4. Dis Model Mech. 2014. PMID: 25190708 Free PMC article.
-
Stromal Lkb1 deficiency leads to gastrointestinal tumorigenesis involving the IL-11-JAK/STAT3 pathway.J Clin Invest. 2018 Jan 2;128(1):402-414. doi: 10.1172/JCI93597. Epub 2017 Dec 4. J Clin Invest. 2018. PMID: 29202476 Free PMC article.
-
LKB1 signaling in advancing cell differentiation.Fam Cancer. 2011 Sep;10(3):425-35. doi: 10.1007/s10689-011-9441-2. Fam Cancer. 2011. PMID: 21519908 Review.
References
-
- Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Hoglund P, Jarvinen H, Kristo P, Pelin K, Ridanpaa M, Salovaara R, Toro T, Bodmer W, Olschwang S, Olsen AS, Stratton MR, de la Chapelle A, Aaltonen LA. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. - PubMed
-
- Hearle N, Schumacher V, Menko FH, Olschwang S, Boardman LA, Gille JJ, Keller JJ, Westerman AM, Scott RJ, Lim W, Trimbath JD, Giardiello FM, Gruber SB, Offerhaus GJ, de Rooij FW, Wilson JH, Hansmann A, Moslein G, Royer-Pokora B, Vogel T, Phillips RK, Spigelman AD, Houlston RS. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res. 2006;12:3209–3215. - PubMed
-
- Baas AF, Smit L, Clevers H. LKB1 tumor suppressor protein: PARtaker in cell polarity. Trends Cell Biol. 2004;14:312–319. - PubMed
-
- Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, Booker SV, Cruz-Correa M, Offerhaus JA. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447–1453. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

