Biochemical characterization of the C4-dicarboxylate transporter DctA from Bacillus subtilis
- PMID: 20363944
- PMCID: PMC2876488
- DOI: 10.1128/JB.00136-10
Biochemical characterization of the C4-dicarboxylate transporter DctA from Bacillus subtilis
Abstract
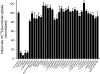
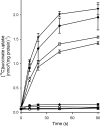
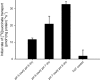
Bacterial secondary transporters of the DctA family mediate ion-coupled uptake of C(4)-dicarboxylates. Here, we have expressed the DctA homologue from Bacillus subtilis in the Gram-positive bacterium Lactococcus lactis. Transport of dicarboxylates in vitro in isolated membrane vesicles was assayed. We determined the substrate specificity, the type of cotransported ions, the electrogenic nature of transport, and the pH and temperature dependence patterns. DctA was found to catalyze proton-coupled symport of the four C(4)-dicarboxylates from the Krebs cycle (succinate, fumurate, malate, and oxaloacetate) but not of other mono- and dicarboxylates. Because (i) succinate-proton symport was electrogenic (stimulated by an internal negative membrane potential) and (ii) the divalent anionic form of succinate was recognized by DctA, at least three protons must be cotransported with succinate. The results were interpreted in the light of the crystal structure of the homologous aspartate transporter Glt(Ph) from Pyrococcus horikoshii.
Figures

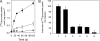
Similar articles
-
A Na+-coupled C4-dicarboxylate transporter (Asuc_0304) and aerobic growth of Actinobacillus succinogenes on C4-dicarboxylates.Microbiology (Reading). 2014 Jul;160(Pt 7):1533-1544. doi: 10.1099/mic.0.076786-0. Epub 2014 Apr 17. Microbiology (Reading). 2014. PMID: 24742960
-
Characterization of the dicarboxylate transporter DctA in Corynebacterium glutamicum.J Bacteriol. 2009 Sep;191(17):5480-8. doi: 10.1128/JB.00640-09. Epub 2009 Jul 6. J Bacteriol. 2009. PMID: 19581365 Free PMC article.
-
Transport of C(4)-dicarboxylates in Wolinella succinogenes.J Bacteriol. 2000 Oct;182(20):5757-64. doi: 10.1128/JB.182.20.5757-5764.2000. J Bacteriol. 2000. PMID: 11004174 Free PMC article.
-
C4-dicarboxylate carriers and sensors in bacteria.Biochim Biophys Acta. 2002 Jan 17;1553(1-2):39-56. doi: 10.1016/s0005-2728(01)00233-x. Biochim Biophys Acta. 2002. PMID: 11803016 Review.
-
Cooperation of Secondary Transporters and Sensor Kinases in Transmembrane Signalling: The DctA/DcuS and DcuB/DcuS Sensor Complexes of Escherichia coli.Adv Microb Physiol. 2016;68:139-67. doi: 10.1016/bs.ampbs.2016.02.003. Epub 2016 Mar 16. Adv Microb Physiol. 2016. PMID: 27134023 Review.
Cited by
-
Mechanism of citrate metabolism by an oxaloacetate decarboxylase-deficient mutant of Lactococcus lactis IL1403.J Bacteriol. 2011 Aug;193(16):4049-56. doi: 10.1128/JB.05012-11. Epub 2011 Jun 10. J Bacteriol. 2011. PMID: 21665973 Free PMC article.
-
Escherichia coli K-12 Transcriptomics for Assessing the Mechanism of Action of High-Power Ultrasound.Microorganisms. 2023 Nov 14;11(11):2768. doi: 10.3390/microorganisms11112768. Microorganisms. 2023. PMID: 38004779 Free PMC article.
-
A comparison of transporter gene expression in three species of Peronospora plant pathogens during host infection.PLoS One. 2023 Jun 1;18(6):e0285685. doi: 10.1371/journal.pone.0285685. eCollection 2023. PLoS One. 2023. PMID: 37262030 Free PMC article.
-
Transporter engineering in microbial cell factories: the ins, the outs, and the in-betweens.Curr Opin Biotechnol. 2020 Dec;66:186-194. doi: 10.1016/j.copbio.2020.08.002. Epub 2020 Sep 12. Curr Opin Biotechnol. 2020. PMID: 32927362 Free PMC article. Review.
-
C4-Dicarboxylate Utilization in Aerobic and Anaerobic Growth.EcoSal Plus. 2016 Jun;7(1):10.1128/ecosalplus.ESP-0021-2015. doi: 10.1128/ecosalplus.ESP-0021-2015. EcoSal Plus. 2016. PMID: 27415771 Free PMC article.
References
-
- Asai, K., S. H. Baik, Y. Kasahara, S. Moriya, and N. Ogasawara. 2000. Regulation of the transport system for C4-dicarboxylic acids in Bacillus subtilis. Microbiology 146(Pt. 2):263-271. - PubMed
-
- Boudker, O., R. M. Ryan, D. Yernool, K. Shimamoto, and E. Gouaux. 2007. Coupling substrate and ion binding to extracellular gate of a sodium-dependent aspartate transporter. Nature 445:387-393. - PubMed
-
- Danbolt, N. C. 2001. Glutamate uptake. Prog. Neurobiol. 65:1-105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

