Loss of enteroendocrine cells in mice alters lipid absorption and glucose homeostasis and impairs postnatal survival
- PMID: 20364088
- PMCID: PMC2860910
- DOI: 10.1172/JCI40794
Loss of enteroendocrine cells in mice alters lipid absorption and glucose homeostasis and impairs postnatal survival
Abstract
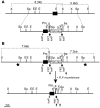
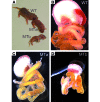
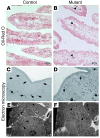
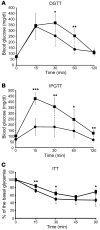
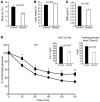
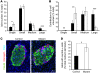
At least 10 enteroendocrine cell types have been identified, and the peptide hormones they secrete have diverse functions that include regulation of glucose homeostasis, food intake, and gastric emptying. Mice lacking individual enteroendocrine hormones, their receptors, or combinations of these have shed light on the role of these hormones in the regulation of energy homeostasis. However, because enteroendocrine hormones have partially overlapping functions, these loss-of-function studies produced only minor phenotypes, and none of the enteroendocrine hormones was shown to be essential for life. To examine the effect of loss of all enteroendocrine cells and hormones on energy homeostasis, we generated mice with intestinal-specific ablation of the proendocrine transcription factor neurogenin 3 (referred to herein as Ngn3Deltaint mice). Ngn3Deltaint mice were deficient for all enteroendocrine cells and hormones, and died with a high frequency during the first week of life. Mutant mice were growth retarded and had yellowish stool suggestive of steatorrhea. Subsequent analyses revealed that Ngn3Deltaint mice had impaired lipid absorption, reduced weight gain, and improved glucose homeostasis. Furthermore, intestinal epithelium of the mutant mice showed an enlarged proliferative crypt compartment and accelerated cell turnover but no changes to goblet and Paneth cell numbers. Enterocytes had shorter microvilli, but the expression of the main brush border enzymes was unaffected. Our data help unravel the role of enteroendocrine cells and hormones in lipid absorption and maintenance of the intestinal epithelium.
Figures
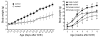





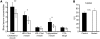

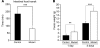
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

