Antiangiogenic cancer therapy combined with oncolytic virotherapy leads to regression of established tumors in mice
- PMID: 20364090
- PMCID: PMC2860921
- DOI: 10.1172/JCI41431
Antiangiogenic cancer therapy combined with oncolytic virotherapy leads to regression of established tumors in mice
Abstract
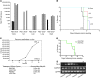
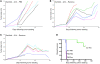
Clinical trials of oncolytic virotherapy have shown low toxicity and encouraging signs of efficacy. However, it remains critically important to develop methods for systemic viral delivery if such therapies are to be clinically implemented to treat established tumors. In this respect, much effort is being focused on combining oncolytic viruses with standard treatment modalities such as inhibitors of VEGF165 (an alternatively spliced isoform of VEGF-A) signaling, which are widely used to treat several different cancers. Here, we have demonstrated that combining VEGF165 inhibitors with systemic delivery of oncolytic viruses leads to substantial regression and cure of established tumors in immunocompetent mice. We have shown that manipulating VEGF165-mediated signaling by administering VEGF165 to mice harboring mouse melanoma cells that do not express VEGF165 and by administering a VEGF inhibitor and then withdrawing treatment to allow VEGF levels to rebound in mice harboring mouse melanoma cells expressing VEGF165 allows tumor-associated endothelial cells transiently to support viral replication. This approach led to direct tumor cell lysis and triggered innate immune-mediated attack on the tumor vasculature. It also resulted in long-term antitumor effects, even against tumors in which viral replication is poorly supported. Since this combinatorial approach targets the tumor endothelium, we believe these data have direct, wide-ranging, and immediate clinical applicability across a broad range of tumor types.
Figures





References
-
- Fisher K. Striking out at disseminated metastases: the systemic delivery of oncolytic viruses. Curr Opin Mol Ther. 2006;8(4):301–313. - PubMed

