The ovary: basic biology and clinical implications
- PMID: 20364094
- PMCID: PMC2846061
- DOI: 10.1172/JCI41350
The ovary: basic biology and clinical implications
Abstract
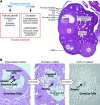
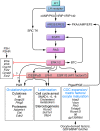
The classical view of ovarian follicle development is that it is regulated by the hypothalamic-pituitary-ovarian axis, in which gonadotropin-releasing hormone (GnRH) controls the release of the gonadotropic hormones follicle-stimulating hormone (FSH) and luteinizing hormone (LH), and that ovarian steroids exert both negative and positive regulatory effects on GnRH secretion. More recent studies in mice and humans indicate that many other intra-ovarian signaling cascades affect follicular development and gonadotropin action in a stage- and context-specific manner. As we discuss here, mutant mouse models and clinical evidence indicate that some of the most powerful intra-ovarian regulators of follicular development include the TGF-beta/SMAD, WNT/FZD/beta-catenin, and RAS/ERK1/2 signaling pathways and the FOXO/FOXL2 transcription factors.
Figures

References
-
- Martins da Silva SJ, Bayne RA, Cambray N, Hartley PS, McNeilly AS, Anderson RA. Expression of activin subunits and receptors in the developing human ovary: activin A promotes germ cell survival and proliferation before primordial follicle formation. Dev Biol. 2004;266(2):334–345. doi: 10.1016/j.ydbio.2003.10.030. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

