Portrait of an oocyte: our obscure origin
- PMID: 20364095
- PMCID: PMC2846057
- DOI: 10.1172/JCI41294
Portrait of an oocyte: our obscure origin
Abstract
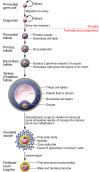
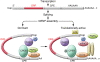
Oocytes play a pivotal role in the cycle of human life. As we discuss here, after emerging from germline stem cells in the fetus, they grow in a follicular niche in which development is harmonized for timely ovulation and hormone secretion after puberty. Most human oocytes have poor developmental competence and are peculiarly vulnerable to chromosomal malsegregation, especially as women pass the optimal years of fertility and may begin to turn to assisted reproductive technologies (ARTs) and egg donation. Research needs to focus on the molecular factors involved and the environmental niche required for optimal development of oocytes, with the aim of increasing their numbers and quality for ARTs, since these are the factors that so often limit human fertility.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

