Fertilization: a sperm's journey to and interaction with the oocyte
- PMID: 20364096
- PMCID: PMC2846064
- DOI: 10.1172/JCI41585
Fertilization: a sperm's journey to and interaction with the oocyte
Abstract
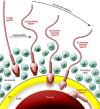
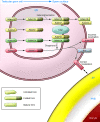
Mammalian fertilization comprises sperm migration through the female reproductive tract, biochemical and morphological changes to sperm, and sperm-egg interaction in the oviduct. Recent gene knockout approaches in mice have revealed that many factors previously considered important for fertilization are largely dispensable, or if they are essential, they have an unexpected function. These results indicate that what has been observed in in vitro fertilization (IVF) differs significantly from what occurs during "physiological" fertilization. This Review focuses on the advantages of studying fertilization using gene-manipulated animals and highlights an emerging molecular mechanism of mammalian fertilization.
Figures


References
-
- Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168(4277):697–698. - PubMed
-
- Austin CR. Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B. 1951;4(4):581–596. - PubMed
-
- Yanagimachi R, Chang MC. Fertilization of hamster eggs in vitro. Nature. 1963;200:281–282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

