Cyclophilin D is required for mitochondrial removal by autophagy in cardiac cells
- PMID: 20364102
- PMCID: PMC3768271
- DOI: 10.4161/auto.6.4.11553
Cyclophilin D is required for mitochondrial removal by autophagy in cardiac cells
Abstract
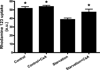
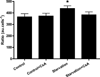
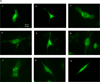
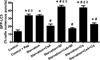
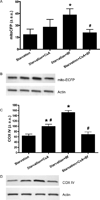
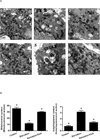
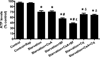
Autophagy is a highly regulated intracellular degradation process by which cells remove cytosolic long-lived proteins and damaged organelles. The mitochondrial permeability transition (MPT) results in mitochondrial depolarization and increased reactive oxygen species production, which can trigger autophagy. Therefore, we hypothesized that the MPT may have a role in signaling autophagy in cardiac cells. Mitochondrial membrane potential was lower in HL-1 cells subjected to starvation compared to cells maintained in full medium. Mitochondrial membrane potential was preserved in starved cells treated with cyclosporin A (CsA), suggesting the MPT pore is associated with starvation-induced depolarization. Starvation-induced autophagy in HL-1 cells, neonatal rat cardiomyocytes and adult mouse cardiomyocytes was inhibited by CsA. Starvation failed to induce autophagy in CypD-deficient murine cardiomyocytes, whereas in myocytes from mice overexpressing CypD the levels of autophagy were enhanced even under fed conditions. Collectively, these results demonstrate a role for CypD and the MPT in the initiation of autophagy. We also analyzed the role of the MPT in the degradation of mitochondria by biochemical analysis and electron microscopy. HL-1 cells subjected to starvation in the presence of CsA had higher levels of mitochondrial proteins (by Western blot), more mitochondria and less autophagosomes (by electron microscopy) than cells starved in the absence of CsA. Our results suggest a physiologic function for CypD and the MPT in the regulation of starvation-induced autophagy. Starvation-induced autophagy regulated by CypD and the MPT may represent a homeostatic mechanism for cellular and mitochondrial quality control.
Conflict of interest statement
DISCLOSURES: None of the authors have any conflicts of interest relevant to this manuscript. RAG is CEO of Radical Therapeutix, Inc.
Figures


References
-
- Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. - PubMed
-
- Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res. 2003;93(4):292–301. - PubMed
-
- Fournier N, Ducet G, Crevat A. Action of cyclosporine on mitochondrial calcium fluxes. J Bioenerg Biomembr. 1987;19:297–303. - PubMed
-
- Nicolli A, Basso E, Petronilli V, Wenger RM, Bernardi P. Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, and cyclosporin A-sensitive channel. J Biol Chem. 1996;271:2185–2192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
