Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss
- PMID: 20364121
- PMCID: PMC2877635
- DOI: 10.1038/nature08894
Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss
Abstract
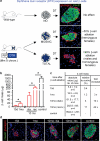
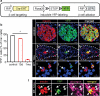
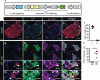
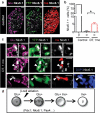
Pancreatic insulin-producing beta-cells have a long lifespan, such that in healthy conditions they replicate little during a lifetime. Nevertheless, they show increased self-duplication after increased metabolic demand or after injury (that is, beta-cell loss). It is not known whether adult mammals can differentiate (regenerate) new beta-cells after extreme, total beta-cell loss, as in diabetes. This would indicate differentiation from precursors or another heterologous (non-beta-cell) source. Here we show beta-cell regeneration in a transgenic model of diphtheria-toxin-induced acute selective near-total beta-cell ablation. If given insulin, the mice survived and showed beta-cell mass augmentation with time. Lineage-tracing to label the glucagon-producing alpha-cells before beta-cell ablation tracked large fractions of regenerated beta-cells as deriving from alpha-cells, revealing a previously disregarded degree of pancreatic cell plasticity. Such inter-endocrine spontaneous adult cell conversion could be harnessed towards methods of producing beta-cells for diabetes therapies, either in differentiation settings in vitro or in induced regeneration.
Figures
Comment in
-
Diabetes forum: Extreme makeover of pancreatic alpha-cells.Nature. 2010 Apr 22;464(7292):1132-3. doi: 10.1038/4641132a. Nature. 2010. PMID: 20414295 Free PMC article.
-
Differentiation: from alpha to beta.Nat Rev Endocrinol. 2010 Aug;6(8):417. doi: 10.1038/nrendo.2010.100. Nat Rev Endocrinol. 2010. PMID: 20681070 No abstract available.
-
GABA signaling: A route to new pancreatic β cells.Cell Res. 2017 Mar;27(3):309-310. doi: 10.1038/cr.2017.20. Epub 2017 Feb 10. Cell Res. 2017. PMID: 28186081 Free PMC article.
References
-
- Zhou Q, Melton DA. Extreme makeover: converting one cell into another. Cell Stem Cell. 2008;3(4):382–388. - PubMed
-
- Uhlenhaut NH, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139(6):1130–1142. - PubMed
-
- Bonal C, et al. Pancreatic Inactivation of c-Myc Decreases Acinar Mass and Transdifferentiates Acinar Cells Into Adipocytes in Mice. Gastroenterology. 2009;136:309–319. - PubMed
-
- Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes. 2005;54(9):2557–2567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

