Import, maturation, and function of SOD1 and its copper chaperone CCS in the mitochondrial intermembrane space
- PMID: 20367259
- PMCID: PMC2962758
- DOI: 10.1089/ars.2010.3212
Import, maturation, and function of SOD1 and its copper chaperone CCS in the mitochondrial intermembrane space
Abstract
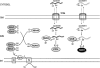
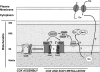
Cu, Zn, superoxide dismutase (SOD1) is a ubiquitous enzyme localized in multiple cellular compartments, including mitochondria, where it concentrates in the intermembrane space (IMS). Similar to other small IMS proteins, the import and retention of SOD1 in the IMS is linked to its folding and maturation, involving the formation of critical intra- and intermolecular disulfide bonds. Therefore, the cysteine residues of SOD1 play a fundamental role in its IMS localization. IMS import of SOD1 involves its copper chaperone, CCS, whose mitochondrial distribution is regulated by the Mia40/Erv1 disulfide relay system in a redox-dependent manner: CCS promotes SOD1 maturation and retention in the IMS. The function of SOD1 in the IMS is still unknown, but it is plausible that it serves to remove superoxide released from the mitochondrial respiratory chain. Mutations in SOD1 cause familial amyotrophic lateral sclerosis (ALS), whose pathologic features include mitochondrial bioenergetic dysfunction. Mutant SOD1 localization in the IMS is not dictated by oxygen concentration and the Mia40/Erv1 system, but is primarily dependent on aberrant protein folding and aggregation. Mutant SOD1 localization and aggregation in the IMS might cause the mitochondrial abnormalities observed in familial ALS and could play a significant role in disease pathogenesis.
Figures
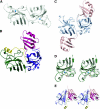
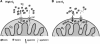
References
-
- Ahmed AU. Fisher PR. Import of nuclear-encoded mitochondrial proteins: a cotranslational perspective. Int Rev Cell Mol Biol. 2009;273:49–68. - PubMed
-
- Arciello M. Capo CR. Cozzolino M. Ferri A. Nencini M. Carri MT. Rossi L. Inactivation of cytochrome c oxidase by mutant SOD1s in mouse motoneuronal NSC-34 cells is independent from copper availability but is because of nitric oxide. J Neurochem. 2010;112:183–192. - PubMed
-
- Arnesano F. Banci L. Bertini I. Martinelli M. Furukawa Y. O'Halloran TV. The unusually stable quaternary structure of human Cu,Zn-superoxide dismutase 1 is controlled by both metal occupancy and disulfide status. J Biol Chem. 2004;279:47998–48003. - PubMed
-
- Bihlmaier K. Mesecke N. Kloeppel C. Herrmann JM. The disulfide relay of the intermembrane space of mitochondria: an oxygen-sensing system? Ann N Y Acad Sci. 2008;1147:293–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

