Proliferation and pluripotency of human embryonic stem cells maintained on type I collagen
- PMID: 20367282
- PMCID: PMC3135251
- DOI: 10.1089/scd.2009.0326
Proliferation and pluripotency of human embryonic stem cells maintained on type I collagen
Abstract
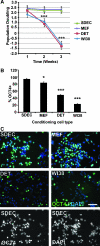
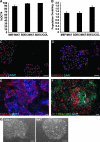
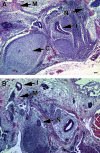
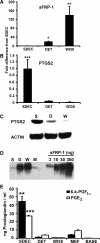
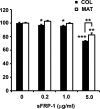
Human embryonic stem cells (hESC) require a balance of growth factors and signaling molecules to proliferate and retain pluripotency. Conditioned medium (CM) from a human embryonic germ-cell-derived cell culture, SDEC, was observed to support the growth of hESC on type I collagen (COL I) and on Matrigel (MAT) biomatricies. After 1 month, the population doubling of hESC grown in SDEC CM on COL I was equivalent to that of hESC grown in mouse embryonic fibroblast (MEF) CM on MAT. hESC grown in SDEC CM on COL I expressed OCT4, NANOG, SSEA-4, alkaline phosphatase (AP), and TRA-1-60; retained a normal karyotype; and were capable of forming teratomas. DNA microarray analysis was used to compare the transcriptional profiles of SDEC and the less supportive WI38 and Detroit 551 human cell lines. The mRNA level of secreted frizzled-related protein (sFRP-1), a known antagonist of the WNT/β-catenin signaling pathway, was significantly reduced in SDEC as compared with the other 2 cell lines, whereas the mRNA levels of prostaglandin-endoperoxide synthase 2 (PTGS2 or COX-2) and prostaglandin I₂ synthase (PGIS), two prostaglandin biosynthesis genes, were significantly increased in SDEC. The level of sFRP-1 protein was significantly reduced, and levels of 2 prostaglandins that are downstream products of PTGS2 and PGIS, prostaglandin E₂ and 6-keto-prostaglandin F(1α), were significantly elevated in SDEC CM compared with WI38, Detroit 551, and MEF CM. Further, addition of purified sFRP-1 to SDEC CM reduced the proliferation of hESC grown on COL I as well as MAT in a dose-dependent manner.
Figures
References
-
- Odorico JS. Kaufman DS. Thomson JA. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19:193–204. - PubMed
-
- Ho HY. Li M. Potential application of embryonic stem cells in Parkinson's disease: drug screening and cell therapy. Regen Med. 2006;1:175–182. - PubMed
-
- Porat S. Dor Y. New sources of pancreatic beta cells. Curr Diab Rep. 2007;7:304–308. - PubMed
-
- Shamblott MJ. Clark GO. Cell therapies for type 1 diabetes mellitus. Expert Opin Biol Ther. 2004;4:269–277. - PubMed
-
- Scharfmann R. Alternative sources of beta cells for cell therapy of diabetes. Eur J Clin Invest. 2003;33:595–600. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

