shRNA-mediated decreases in c-Met levels affect the differentiation potential of human mesenchymal stem cells and reduce their capacity for tissue repair
- PMID: 20367286
- PMCID: PMC2947453
- DOI: 10.1089/ten.TEA.2009.0363
shRNA-mediated decreases in c-Met levels affect the differentiation potential of human mesenchymal stem cells and reduce their capacity for tissue repair
Abstract
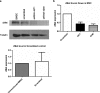
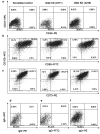
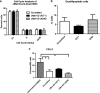
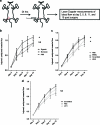
Mesenchymal stem cells/marrow stromal cells (MSC) are adult multipotent cells that can augment tissue repair. We previously demonstrated that culturing MSC in hypoxic conditions causes upregulation of the hepatocyte growth factor (HGF) receptor c-Met, allowing them to respond more robustly to HGF. MSC preconditioned in hypoxic environments contributed to restoration of blood flow after an ischemic injury more rapidly than MSC cultured in normoxic conditions. We now investigated the specific role of HGF/c-Met signaling in MSC function. An shRNA-mediated knockdown (KD) of c-Met in MSC did not alter their phenotypic profile, proliferation, or viability in vitro. However, we determined that while HGF/c-Met signaling does not play a role in the adipogenic differentiation of the cells, the disruption of this signaling pathway inhibited the ability of MSC to differentiate into the osteogenic and chondrogenic lineages. We next assessed the impact of c-Met KD on human MSC function in a xenogeneic hindlimb ischemia injury model. A 70% KD of c-Met in MSC resulted in a significant decrease in their capacity to regenerate blood flow to the ischemic limb, as compared to the MSC transduced with control shRNA. MSC with only a 60% KD of c-Met exhibited an intermediate capacity to restore blood flow, suggesting that MSC function is sensitive to the dosage of c-Met signaling. The current study highlights the significance of HGF/c-Met signaling in the capacity of MSC to restore blood flow after an ischemic injury and in their ability to differentiate into the osteogenic and chondrogenic lineages.
Figures

Similar articles
-
Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells.Stem Cells. 2008 Aug;26(8):2173-82. doi: 10.1634/stemcells.2007-1104. Epub 2008 May 29. Stem Cells. 2008. PMID: 18511601 Free PMC article.
-
C-Met-Activated Mesenchymal Stem Cells Rescue Ischemic Damage via Interaction with Cellular Prion Protein.Cell Physiol Biochem. 2018;46(5):1835-1848. doi: 10.1159/000489368. Epub 2018 Apr 25. Cell Physiol Biochem. 2018. PMID: 29705776
-
Enhancement of angiogenic effects by hypoxia-preconditioned human umbilical cord-derived mesenchymal stem cells in a mouse model of hindlimb ischemia.Cell Biol Int. 2016 Jan;40(1):27-35. doi: 10.1002/cbin.10519. Epub 2015 Aug 17. Cell Biol Int. 2016. PMID: 26222206
-
sFRP2 suppression of bone morphogenic protein (BMP) and Wnt signaling mediates mesenchymal stem cell (MSC) self-renewal promoting engraftment and myocardial repair.J Biol Chem. 2010 Nov 12;285(46):35645-53. doi: 10.1074/jbc.M110.135335. Epub 2010 Sep 7. J Biol Chem. 2010. PMID: 20826809 Free PMC article.
-
Musculoskeletal Progenitor/Stromal Cell-Derived Mitochondria Modulate Cell Differentiation and Therapeutical Function.Front Immunol. 2021 Mar 8;12:606781. doi: 10.3389/fimmu.2021.606781. eCollection 2021. Front Immunol. 2021. PMID: 33763061 Free PMC article. Review.
Cited by
-
Effects on proliferation and differentiation of multipotent bone marrow stromal cells engineered to express growth factors for combined cell and gene therapy.Stem Cells. 2011 Nov;29(11):1727-37. doi: 10.1002/stem.720. Stem Cells. 2011. PMID: 21898687 Free PMC article.
-
Characterization and in vivo testing of mesenchymal stem cells derived from human embryonic stem cells.Tissue Eng Part A. 2011 Jun;17(11-12):1517-25. doi: 10.1089/ten.TEA.2010.0460. Epub 2011 Mar 4. Tissue Eng Part A. 2011. PMID: 21275830 Free PMC article.
-
Bottom-up signaling from HGF-containing surfaces promotes hepatic differentiation of mesenchymal stem cells.Biochem Biophys Res Commun. 2011 Apr 8;407(2):295-300. doi: 10.1016/j.bbrc.2011.03.005. Epub 2011 Mar 5. Biochem Biophys Res Commun. 2011. PMID: 21382341 Free PMC article.
-
Cell Therapy and Critical Limb Ischemia: Evidence and Window of Opportunity in Obesity.Obes Control Ther. 2016;3(1):121. doi: 10.15226/2374-8354/3/1/00121. Epub 2016 Sep 15. Obes Control Ther. 2016. PMID: 28979948 Free PMC article. No abstract available.
-
Therapeutic potential for mesenchymal stem cell transplantation in critical limb ischemia.Stem Cell Res Ther. 2012 Jul 30;3(4):28. doi: 10.1186/scrt119. Stem Cell Res Ther. 2012. PMID: 22846185 Free PMC article. Review.
References
-
- Horwitz E.M. Le Blanc K. Dominici M. Mueller I. Slaper-Cortenbach I. Marini F.C. Deans R.J. Krause D.S. Keating A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393. - PubMed
-
- Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. - PubMed
-
- Sanchez-Ramos J. Song S. Cardozo-Pelaez F. Hazzi C. Stedeford T. Willing A. Freeman T.B. Saporta S. Janssen W. Patel N. Cooper D.R. Sanberg P.R. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

