When NRF2 talks, who's listening?
- PMID: 20367496
- PMCID: PMC2966480
- DOI: 10.1089/ars.2010.3216
When NRF2 talks, who's listening?
Abstract
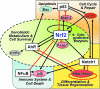
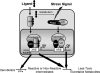
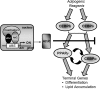
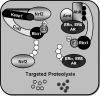
Activation of the KEAP1-NRF2 signaling pathway is an adaptive response to environmental and endogenous stresses and serves to render animals resistant to chemical carcinogenesis and other forms of toxicity, whereas disruption of the pathway exacerbates these outcomes. This pathway, which can be activated by sulfhydryl-reactive, small-molecule pharmacologic agents, regulates the inducible expression of an extended battery of cytoprotective genes, often by direct binding of the transcription factor to antioxidant response elements in the promoter regions of target genes. However, it is becoming evident that some of the protective effects may be mediated indirectly through cross talk with additional pathways affecting cell survival and other aspects of cell fate. These interactions provide a multi-tiered, integrated response to chemical stresses. This review highlights recent observations on the molecular interactions and their functional consequences between NRF2 and the arylhydrocarbon receptor (AhR), NF-κB, p53, and Notch1 signaling pathways.
Figures


Similar articles
-
Regulation of notch1 signaling by nrf2: implications for tissue regeneration.Sci Signal. 2010 Jul 13;3(130):ra52. doi: 10.1126/scisignal.2000762. Sci Signal. 2010. PMID: 20628156 Free PMC article.
-
Molecular cross-talk between the NRF2/KEAP1 signaling pathway, autophagy, and apoptosis.Free Radic Biol Med. 2011 May 1;50(9):1186-95. doi: 10.1016/j.freeradbiomed.2011.01.033. Epub 2011 Feb 2. Free Radic Biol Med. 2011. PMID: 21295136
-
Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway.Antioxid Redox Signal. 2010 Dec 1;13(11):1713-48. doi: 10.1089/ars.2010.3221. Epub 2010 Aug 14. Antioxid Redox Signal. 2010. PMID: 20446772 Review.
-
NRF2 modulates aryl hydrocarbon receptor signaling: influence on adipogenesis.Mol Cell Biol. 2007 Oct;27(20):7188-97. doi: 10.1128/MCB.00915-07. Epub 2007 Aug 20. Mol Cell Biol. 2007. PMID: 17709388 Free PMC article.
-
Nrf2 protects against airway disorders.Toxicol Appl Pharmacol. 2010 Apr 1;244(1):43-56. doi: 10.1016/j.taap.2009.07.024. Epub 2009 Jul 29. Toxicol Appl Pharmacol. 2010. PMID: 19646463 Review.
Cited by
-
Differential sensitivity to pro-oxidant exposure in two populations of killifish (Fundulus heteroclitus).Ecotoxicology. 2013 Mar;22(2):387-401. doi: 10.1007/s10646-012-1033-x. Epub 2013 Jan 18. Ecotoxicology. 2013. PMID: 23329125 Free PMC article.
-
Nrf2: a potential target for new therapeutics in liver disease.Clin Pharmacol Ther. 2012 Sep;92(3):340-8. doi: 10.1038/clpt.2012.110. Epub 2012 Aug 8. Clin Pharmacol Ther. 2012. PMID: 22871994 Free PMC article. Review.
-
Shikonin Induces Apoptotic Cell Death via Regulation of p53 and Nrf2 in AGS Human Stomach Carcinoma Cells.Biomol Ther (Seoul). 2016 Sep 1;24(5):501-9. doi: 10.4062/biomolther.2016.008. Biomol Ther (Seoul). 2016. PMID: 27257011 Free PMC article.
-
CD137 Signaling Promotes Endothelial Apoptosis by Inhibiting Nrf2 Pathway, and Upregulating NF-κB Pathway.Mediators Inflamm. 2020 Jun 6;2020:4321912. doi: 10.1155/2020/4321912. eCollection 2020. Mediators Inflamm. 2020. PMID: 32587470 Free PMC article.
-
Resveratrol protects C6 astrocyte cell line against hydrogen peroxide-induced oxidative stress through heme oxygenase 1.PLoS One. 2013 May 15;8(5):e64372. doi: 10.1371/journal.pone.0064372. Print 2013. PLoS One. 2013. PMID: 23691207 Free PMC article.
References
-
- Ahmad R. Raina D. Meyer C. Kharbanda S. Kufe D. Triterpenoid CDDO-Me blocks the NF-kappaB pathway by direct inhibition of IKKbeta on Cys-179. J Biol Chem. 2006;281:35764–35769. - PubMed
-
- Ahn KS. Aggarwal BB. Transcription factor NF-kappaB: a sensor for smoke and stress signals. Ann N Y Acad Sci. 2005;1056:218–2133. - PubMed
-
- Alam J. Stewart D. Touchard C. Boinapally S. Choi AM. Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999;274:26071–26078. - PubMed
-
- Albakri QA. Stuehr DJ. Intracellular assembly of inducible NO synthase is limited by nitric oxide-mediated changes in heme insertion and availability. J Biol Chem. 1996;271:5414–5421. - PubMed
-
- Alexander DL. Ganem LG. Fernandez-Salguero P. Gonzalez F. Jefcoate CR. Aryl-hydrocarbon receptor is an inhibitory regulator of lipid synthesis and of commitment to adipogenesis. J Cell Sci. 1998;111:3311–3322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

