Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens
- PMID: 20367623
- PMCID: PMC3837356
- DOI: 10.1111/j.1600-0897.2010.00842.x
Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens
Abstract
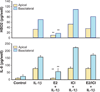
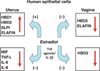
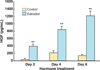
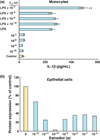
The immune system in the female reproductive tract (FRT) does not mount an attack against HIV or other sexually transmitted infections (STI) with a single endogenously produced microbicide or with a single arm of the immune system. Instead, the body deploys dozens of innate antimicrobials to the secretions of the female reproductive tract. Working together, these antimicrobials along with mucosal antibodies attack many different viral, bacterial and fungal targets. Within the FRT, the unique challenges of protection against sexually transmitted pathogens coupled with the need to sustain the development of an allogeneic fetus have evolved in such a way that sex hormones precisely regulate immune function to accomplish both tasks. The studies presented in this review demonstrate that estradiol and progesterone secreted during the menstrual cycle act both directly and indirectly on epithelial cells and other immune cells in the reproductive tract to modify immune function in a way that is unique to specific sites throughout the FRT. As presented in this review, studies from our laboratory and others demonstrate that the innate immune response is under hormonal control, varies with the stage of the menstrual cycle, and as such is suppressed at mid-cycle to optimize conditions for successful fertilization and pregnancy. In doing so, a window of STI vulnerability is created during which potential pathogens including HIV enter the reproductive tract to infect host targets.
Figures

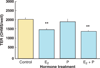
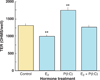
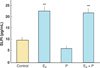
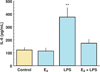


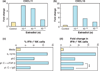
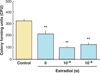
References
-
- UNAIDS 2007. AIDS epidemic update. Geneva, Switzerland: JUNPoHAUaWHOW; 2007.
-
- Ogra P, Yamanaka T, Losonsky GA. Local immunologic defenses in the genital tract. In: Fleicher N, editor. Local Immunologic Defenses in the Genital Tract. New York: Alan R. Liss, Inc.; 1981. pp. 381–394. - PubMed
-
- Wira CR, Fahey JV, White HD, Yeaman GR, Given AL, Howell AL. The mucosal immune system in the human female reproductive tract: influence of stage of the menstrual cycle and menopause on mucosal immunity in the uterus. In: Glasser S, Aplin J, Guidice L, Tabibzadeh S, editors. The Mucosal Immune System in the Human Female Reproductive Tract: influence of Stage of the Menstrual Cycle and Menopause on Mucosal Immunity in the Uterus. New York: Taylor and Francis; 2002. pp. 371–404.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

